1. A platinum (II) compound , which is used to treat tumors, contains 65.0% Pt., 23.6% Cl, 9.35% N, and 2.05% H by mass. Calculate its empirical formula.
1. A platinum (II) compound , which is used to treat tumors, contains 65.0% Pt., 23.6% Cl, 9.35% N, and 2.05% H by mass. Calculate its empirical formula.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.75PAE: 3.75 The following pictures show a molecular-scale view of a chemical reaction between the compounds...
Related questions
Question
I. Solve the following problems: Show your solutions.
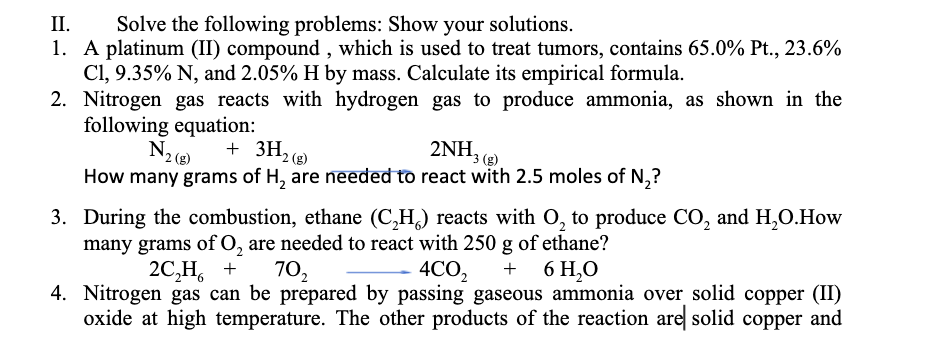
Transcribed Image Text:II.
Solve the following problems: Show your solutions.
1. A platinum (II) compound , which is used to treat tumors, contains 65.0% Pt., 23.6%
Cl, 9.35% N, and 2.05% H by mass. Calculate its empirical formula.
2. Nitrogen gas reacts with hydrogen gas to produce ammonia, as shown in the
following equation:
+ 3H2 (8)
2ΝH,
3 (g)
How many grams of H, are needed to react with 2.5 moles of N,?
3. During the combustion, ethane (C,H,) reacts with O, to produce CO, and H,O.How
many grams of O, are needed to react with 250 g of ethane?
2C,Н, +
4CO2
4. Nitrogen gas can be prepared by passing gaseous ammonia over solid copper (II)
oxide at high temperature. The other products of the reaction are solid copper and
70,
6 H,O
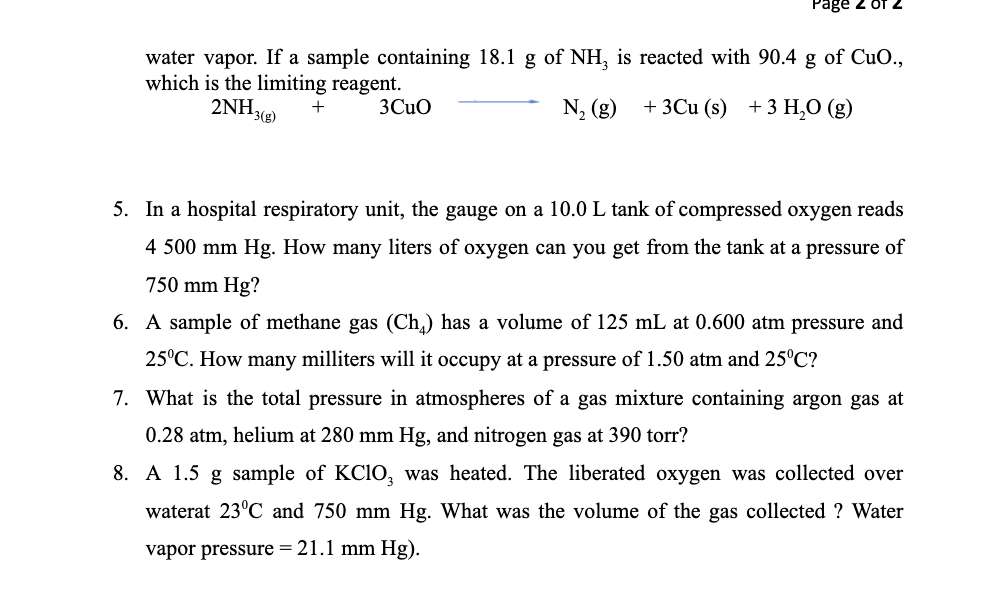
Transcribed Image Text:Page 2 of
water vapor. If a sample containing 18.1 g of NH, is reacted with 90.4 g of CuO.,
which is the limiting reagent.
2NH3(g)
N, (g)
+ 3Cu (s) +3 H,O (g)
+
3Cuo
5. In a hospital respiratory unit, the gauge on a 10.0 L tank of compressed oxygen reads
4 500 mm Hg. How many liters of oxygen can you get from the tank at a pressure of
750 mm Hg?
6. A sample of methane gas (Ch) has a volume of 125 mL at 0.600 atm pressure and
25°C. How many milliters will it occupy at a pressure of 1.50 atm and 25°C?
7. What is the total pressure in atmospheres of a gas mixture containing argon gas at
0.28 atm, helium at 280 mm Hg, and nitrogen gas at 390 torr?
8. A 1.5 g sample of KCIO, was heated. The liberated oxygen was collected over
waterat 23°C and 750 mm Hg. What was the volume of the gas collected ? Water
vapor pressure = 21.1 mm Hg).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning