1. a) True or False? For a reaction at equilibrium, the value of AG is 0, which means that the process is not spontaneous in either direction. b) True or False? Catalysts accelerate chemical reactions by changing the mechanism and lowering E,, but they are consumed or destroyed in the process. c) True or False? Enzymes (like alcohol dehydrogenase, which removes ethanol from our bodies) universally show zero order kinetics. d) True or False? For a reaction to be successful, molecules must collide with appropriate activation energy (E,), but their orientation with respect to one another is not important. e) True or False? The collisional frequency for 2 reactants increases as their concentration decreases.
1. a) True or False? For a reaction at equilibrium, the value of AG is 0, which means that the process is not spontaneous in either direction. b) True or False? Catalysts accelerate chemical reactions by changing the mechanism and lowering E,, but they are consumed or destroyed in the process. c) True or False? Enzymes (like alcohol dehydrogenase, which removes ethanol from our bodies) universally show zero order kinetics. d) True or False? For a reaction to be successful, molecules must collide with appropriate activation energy (E,), but their orientation with respect to one another is not important. e) True or False? The collisional frequency for 2 reactants increases as their concentration decreases.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 5ALQ
Related questions
Question

Transcribed Image Text:c) What's the sign of AS in the event: HCI (g) –> HCI (aq)? Why?.
d) Consider if a minor earthquake near TO rattled the books off shelves in the campus library.
If the library were considered the system, what is its sign for AS?
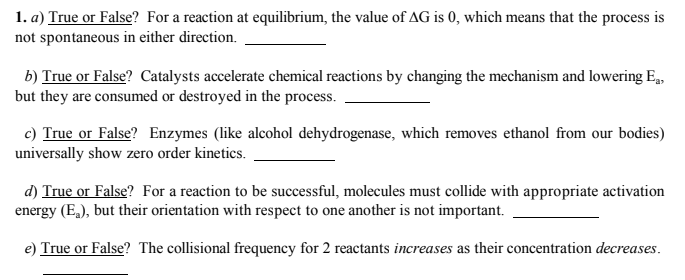
Transcribed Image Text:1. a) True or False? For a reaction at equilibrium, the value of AG is 0, which means that the process is
not spontaneous in either direction.
b) True or False? Catalysts accelerate chemical reactions by changing the mechanism and lowering E,
but they are consumed or destroyed in the process.
c) True or False? Enzymes (like alcohol dehydrogenase, which removes ethanol from our bodies)
universally show zero order kinetics.
d) True or False? For a reaction to be successful, molecules must collide with appropriate activation
energy (E,), but their orientation with respect to one another is not important.
e) True or False? The collisional frequency for 2 reactants increases as their concentration decreases.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning