1. a. Write the balanced chemical equation for the reaction b. Using the curly arrow notation draw a mechanism for the reaction c. Explain the role of cHCI in the work-up step
1. a. Write the balanced chemical equation for the reaction b. Using the curly arrow notation draw a mechanism for the reaction c. Explain the role of cHCI in the work-up step
Chapter89: Thin-layer Chromatography
Section: Chapter Questions
Problem 5P
Related questions
Question
Reduction of Benzophenone by Sodium borohydride.
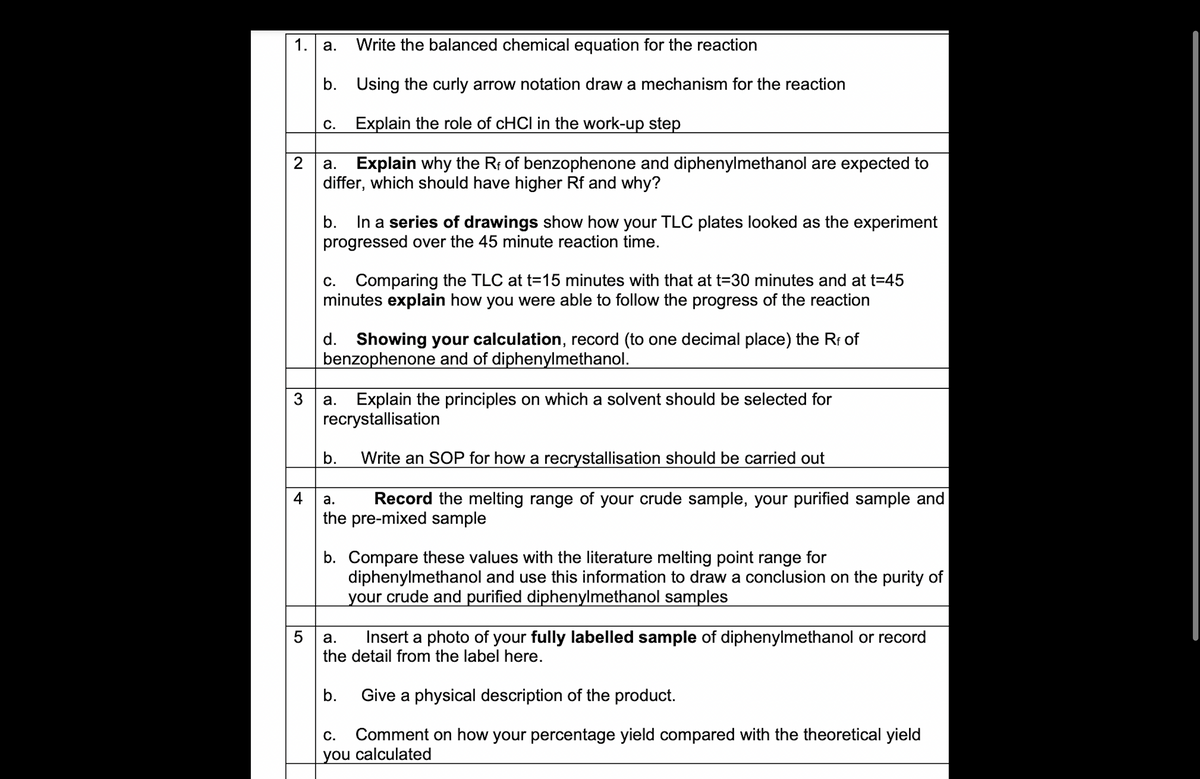
Transcribed Image Text:1.
a. Write the balanced chemical equation for the reaction
b. Using the curly arrow notation draw a mechanism for the reaction
Explain the role of cHCI in the work-up step
2
a. Explain why the Rf of benzophenone and diphenylmethanol are expected to
differ, which should have higher Rf and why?
C.
4
b. In a series of drawings show how your TLC plates looked as the experiment
progressed over the 45 minute reaction time.
c. Comparing the TLC at t=15 minutes with that at t=30 minutes and at t=45
minutes explain how you were able to follow the progress of the reaction
d. Showing your calculation, record (to one decimal place) the Rf of
benzophenone and of diphenylmethanol.
3
a. Explain the principles on which a solvent should be selected for
recrystallisation
Write an SOP for how a recrystallisation should be carried out
a.
Record the melting range of your crude sample, your purified sample and
the pre-mixed sample
b.
b. Compare these values with the literature melting point range for
diphenylmethanol and use this information to draw a conclusion on the purity of
your crude and purified diphenylmethanol samples
5
a. Insert a photo of your fully labelled sample of diphenylmethanol or record
the detail from the label here.
Give a physical description of the product.
C. Comment on how your percentage yield compared with the theoretical yield
you calculated
b.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole