1. Ammonia, NH3, is prepared by the following reaction: 2 NO2(g) + 5 H2(g) - 2 NH3(g) + 2 H2O(I) Calculate the percentage yield of ammonia in a reaction of 45.8g of NO and 12.4 g of H2? Show all the steps to obtain the final answer. 2. PBrs is prepared according to the following reaction: PBr3 + Br2 PBrs When 65.6 g of Br2 reacts with excess PB13, 125.2 g of PBrs is produced. Calculate the percentage yield for the reaction.
1. Ammonia, NH3, is prepared by the following reaction: 2 NO2(g) + 5 H2(g) - 2 NH3(g) + 2 H2O(I) Calculate the percentage yield of ammonia in a reaction of 45.8g of NO and 12.4 g of H2? Show all the steps to obtain the final answer. 2. PBrs is prepared according to the following reaction: PBr3 + Br2 PBrs When 65.6 g of Br2 reacts with excess PB13, 125.2 g of PBrs is produced. Calculate the percentage yield for the reaction.
An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter14: Organic Chemistry
Section: Chapter Questions
Problem 2AYK: You overhear someone comment that a lot of cat cracking takes place in oil refineries. Should you...
Related questions
Question
100%
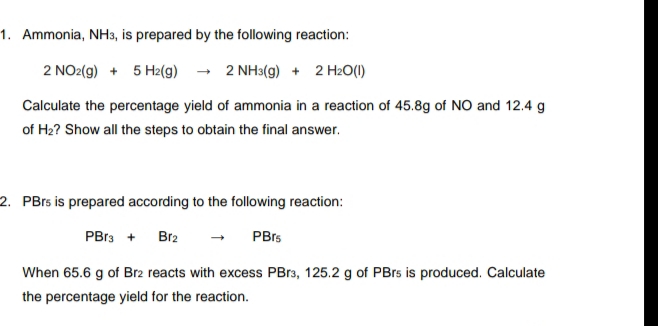
Transcribed Image Text:1. Ammonia, NH3, is prepared by the following reaction:
2 NO2(g) + 5 H2(g) - 2 NH3(g) + 2 H2O(I)
Calculate the percentage yield of ammonia in a reaction of 45.8g of NO and 12.4 g
of H2? Show all the steps to obtain the final answer.
2. PBrs is prepared according to the following reaction:
PBr3 +
Br2
PBrs
When 65.6 g of Br2 reacts with excess PB13, 125.2 g of PBrs is produced. Calculate
the percentage yield for the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning
