1. Calculate the mass percent, % (m/m), for the solute in the following: 24.0 g of KCI and 200. g of H2O. Do not include the percent symbol, just report the number, watch sig figs. 2. How many grams of solute are needed to make 1300 mL of a 4.5% (m/v) NH4Cl solution? Do not include units in your answer. 3. How many milliliters of solute are needed to make 840. mL of a 16.5% (v/v) acetic acid solution? Do not include units in your answer. 4. Calculate the mass percent, % (m/m), for the solute in the following: 12 g of KCI and 250 g of tea with sugar (solution). Do not include the percent symbol, just report the number, watch sig figs. 5. Calculate the volume in milliliters of a 2.200 M K2SO4 solution to obtain 3.000 mol of K2SO4. Do not include units. Ο o 945 =
1. Calculate the mass percent, % (m/m), for the solute in the following: 24.0 g of KCI and 200. g of H2O. Do not include the percent symbol, just report the number, watch sig figs. 2. How many grams of solute are needed to make 1300 mL of a 4.5% (m/v) NH4Cl solution? Do not include units in your answer. 3. How many milliliters of solute are needed to make 840. mL of a 16.5% (v/v) acetic acid solution? Do not include units in your answer. 4. Calculate the mass percent, % (m/m), for the solute in the following: 12 g of KCI and 250 g of tea with sugar (solution). Do not include the percent symbol, just report the number, watch sig figs. 5. Calculate the volume in milliliters of a 2.200 M K2SO4 solution to obtain 3.000 mol of K2SO4. Do not include units. Ο o 945 =
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 6QAP
Related questions
Question
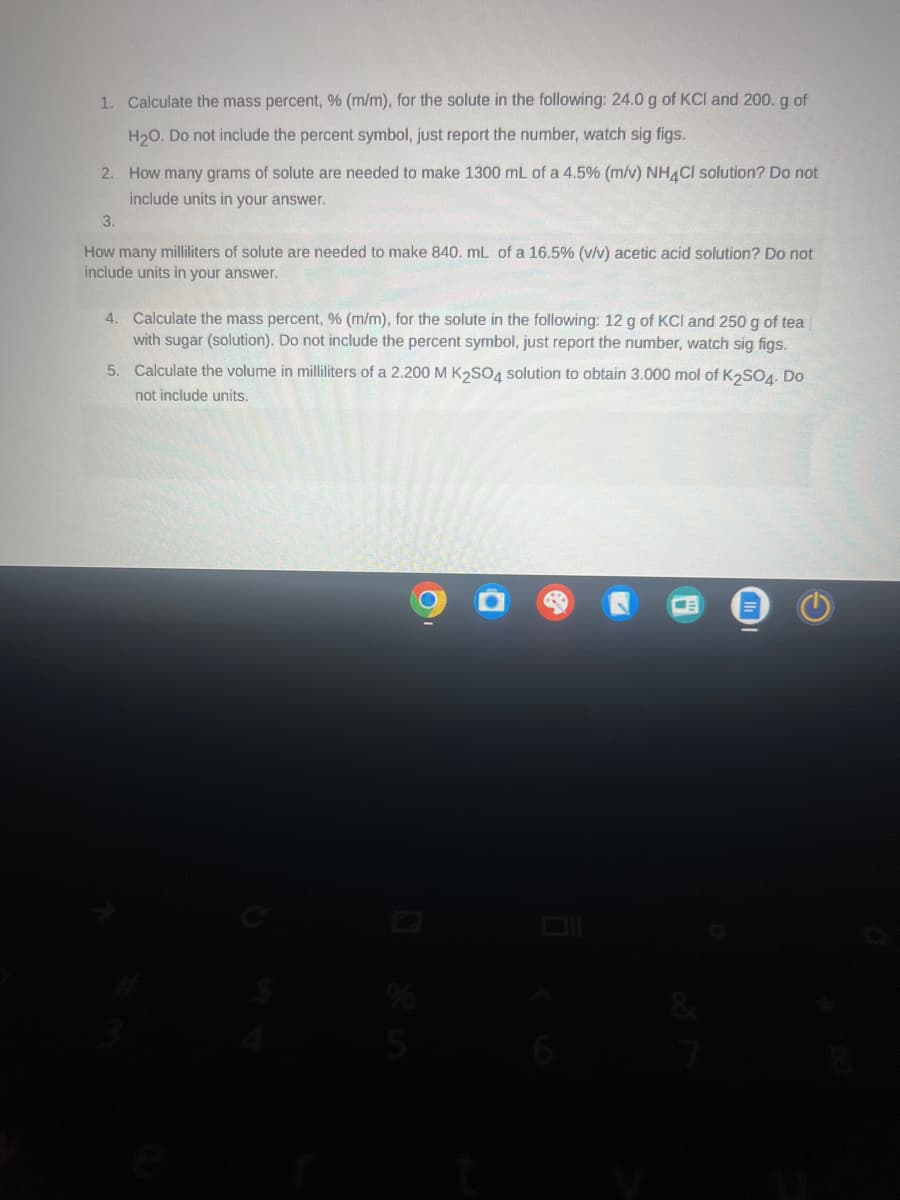
Transcribed Image Text:1. Calculate the mass percent, % (m/m), for the solute in the following: 24.0 g of KCI and 200. g of
H2O. Do not include the percent symbol, just report the number, watch sig figs.
2. How many grams of solute are needed to make 1300 mL of a 4.5% (m/v) NH4Cl solution? Do not
include units in your answer.
3.
How many milliliters of solute are needed to make 840. mL of a 16.5% (v/v) acetic acid solution? Do not
include units in your answer.
4. Calculate the mass percent, % (m/m), for the solute in the following: 12 g of KCI and 250 g of tea
with sugar (solution). Do not include the percent symbol, just report the number, watch sig figs.
5. Calculate the volume in milliliters of a 2.200 M K2SO4 solution to obtain 3.000 mol of K2SO4. Do
not include units.
Ο
o
945
=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning
