Hydrogen peroxide (34.02 g/mol) decomposes into water and oxygen gas according to the reaction 2 H₂O2 (1) → 2 H2O (l) + O2 (g) A 7.50 g sample of hydrogen peroxide is sealed in a 375 mL container at 350 K. Identify the strategy you would use to determine the pressure of O2 gas produced from the decomposition of the hydrogen peroxide sample. -> grams H2O2 → moles H2O2 → moles O₂ → pressure 02 → moles 02 → pressure O2 → grams H2O2 moles H2O2 pressure 02 → moles 02 → moles H2O2 · →> grams H2O2 moles H2O2 → pressure 02
Hydrogen peroxide (34.02 g/mol) decomposes into water and oxygen gas according to the reaction 2 H₂O2 (1) → 2 H2O (l) + O2 (g) A 7.50 g sample of hydrogen peroxide is sealed in a 375 mL container at 350 K. Identify the strategy you would use to determine the pressure of O2 gas produced from the decomposition of the hydrogen peroxide sample. -> grams H2O2 → moles H2O2 → moles O₂ → pressure 02 → moles 02 → pressure O2 → grams H2O2 moles H2O2 pressure 02 → moles 02 → moles H2O2 · →> grams H2O2 moles H2O2 → pressure 02
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.129QP: Carbon monoxide, CO, and oxygen, O2, react according to 2CO(g)+O2(g)2CO2(g) Assuming that the...
Related questions
Question
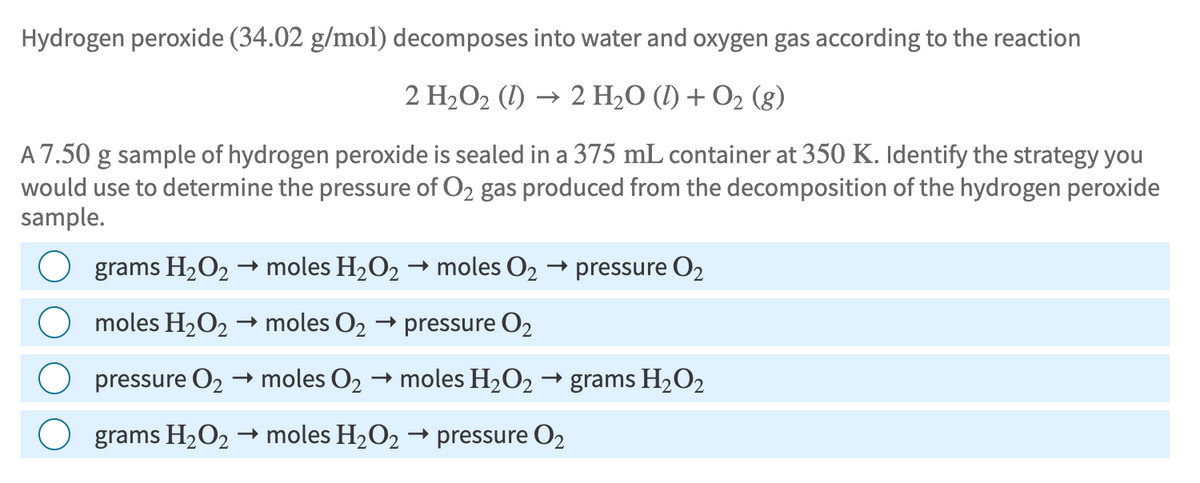
Transcribed Image Text:Hydrogen peroxide (34.02 g/mol) decomposes into water and oxygen gas according to the reaction
2 H₂O2 (1) → 2 H2O (l) + O2 (g)
A 7.50 g sample of hydrogen peroxide is sealed in a 375 mL container at 350 K. Identify the strategy you
would use to determine the pressure of O2 gas produced from the decomposition of the hydrogen peroxide
sample.
->
grams H2O2 → moles H2O2 → moles O₂
→ pressure 02
→ moles 02 → pressure O2
→ grams H2O2
moles H2O2
pressure 02 → moles 02 → moles H2O2 ·
→>
grams H2O2 moles H2O2 → pressure 02
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning