1. Dehydration: e • 2. Oxidation: ● ● ● ALCOHOLS This is a reaction where an alcohol loses a water molecule to form an alkene. For example, when ethanol is treated with an acidic catalyst, such as sulfuric acid, it undergoes dehydration to form ethene (CH2=CH2) and water. Types of Reaction(s) In this reaction, an alcohol is converted to either a carbonyl compound or a carboxylic acid. For example, primary alcohols can be oxidized to aldehydes or carboxylic acids, while secondary alcohols can be oxidized to ketones. Tertiary alcohols are usually not affected by oxidations 3. Esterification: ● The conversion of an alcohol and a carboxylic acid to an ester and water, in the presence of an acid catalyst. The reaction between methanol and acetic acid to form methyl acetate: CH3OH + CH3COOH CH3COOCH3 + H2O 4. Substitution: . ● Substitution reactions in organic chemistry involve the replacement of one atom or group of atoms with another atom or group of atoms in a molecule. 1. Cleavage reactions: ● 2. Williamson ether synthesis: ● ● Ethers can be cleaved by strong acids, such as HBr or HI, to form alkyl halides and alcohols. 3. Acid-catalyzed dehydration: ● ETHERS ● This is a method for the synthesis of ethers by the reaction of an alkoxide ion with an alkyl halide. 4. Oxidation: An ether is converted into an alkene by the elimination of a water molecule under acidic conditions. Ethers can be oxidized with strong oxidizing agents, such as peracids or chromium reagents, to form carbonyl compounds. However, ethers are generally less prone to oxidation compared to alcohols
1. Dehydration: e • 2. Oxidation: ● ● ● ALCOHOLS This is a reaction where an alcohol loses a water molecule to form an alkene. For example, when ethanol is treated with an acidic catalyst, such as sulfuric acid, it undergoes dehydration to form ethene (CH2=CH2) and water. Types of Reaction(s) In this reaction, an alcohol is converted to either a carbonyl compound or a carboxylic acid. For example, primary alcohols can be oxidized to aldehydes or carboxylic acids, while secondary alcohols can be oxidized to ketones. Tertiary alcohols are usually not affected by oxidations 3. Esterification: ● The conversion of an alcohol and a carboxylic acid to an ester and water, in the presence of an acid catalyst. The reaction between methanol and acetic acid to form methyl acetate: CH3OH + CH3COOH CH3COOCH3 + H2O 4. Substitution: . ● Substitution reactions in organic chemistry involve the replacement of one atom or group of atoms with another atom or group of atoms in a molecule. 1. Cleavage reactions: ● 2. Williamson ether synthesis: ● ● Ethers can be cleaved by strong acids, such as HBr or HI, to form alkyl halides and alcohols. 3. Acid-catalyzed dehydration: ● ETHERS ● This is a method for the synthesis of ethers by the reaction of an alkoxide ion with an alkyl halide. 4. Oxidation: An ether is converted into an alkene by the elimination of a water molecule under acidic conditions. Ethers can be oxidized with strong oxidizing agents, such as peracids or chromium reagents, to form carbonyl compounds. However, ethers are generally less prone to oxidation compared to alcohols
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter21: Organic And Biological Molecules
Section: Chapter Questions
Problem 5RQ: What functional group distinguishes each of the following hydrocarbon derivatives? a....
Related questions
Question
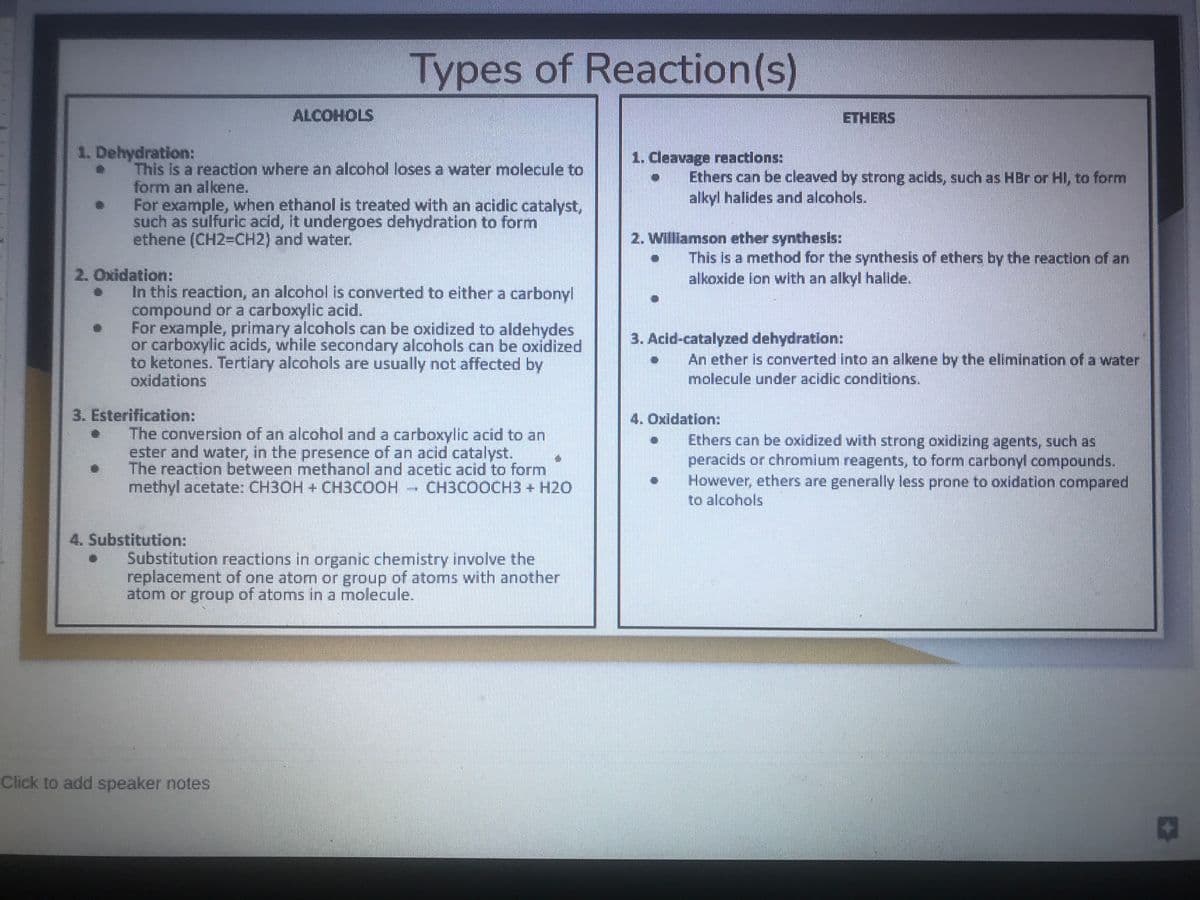
Transcribed Image Text:1. Dehydration:
2. Oxidation:
This is a reaction where an alcohol loses a water molecule to
form an alkene.
For example, when ethanol is treated with an acidic catalyst,
such as sulfuric acid, it undergoes dehydration to form
ethene (CH2=CH2) and water.
ALCOHOLS
In this reaction, an alcohol is converted to either a carbonyl
compound or a carboxylic acid.
3. Esterification:
Types of Reaction (s)
For example, primary alcohols can be oxidized to aldehydes
or carboxylic acids, while secondary alcohols can be oxidized
to ketones. Tertiary alcohols are usually not affected by
oxidations
4. Substitution:
The conversion of an alcohol and a carboxylic acid to an
ester and water, in the presence of an acid catalyst.
The reaction between methanol and acetic acid to form
methyl acetate: CH3OH + CH3COOH CH3COOCH3 + H2O
Click to add speaker notes
ME
Substitution reactions in organic chemistry involve the
replacement of one atom or group of atoms with another
atom or group of atoms in a molecule.
1. Cleavage reactions:
Ethers can be cleaved by strong acids, such as HBr or HI, to form
alkyl halides and alcohols.
2. Williamson ether synthesis:
ETHERS
This is a method for the synthesis of ethers by the reaction of an
alkoxide ion with an alkyl halide.
3. Acid-catalyzed dehydration:
An ether is converted into an alkene by the elimination of a water
molecule under acidic conditions.
4. Oxidation:
Ethers can be oxidized with strong oxidizing agents, such as
peracids or chromium reagents, to form carbonyl compounds.
However, ethers are generally less prone to oxidation compared
to alcohols

Transcribed Image Text:1. Dehydration:
2. Oxidation:
This is a reaction where an alcohol loses a water molecule to
form an alkene.
For example, when ethanol is treated with an acidic catalyst,
such as sulfuric acid, it undergoes dehydration to form
ethene (CH2=CH2) and water.
ALCOHOLS
In this reaction, an alcohol is converted to either a carbonyl
compound or a carboxylic acid.
3. Esterification:
Types of Reaction (s)
For example, primary alcohols can be oxidized to aldehydes
or carboxylic acids, while secondary alcohols can be oxidized
to ketones. Tertiary alcohols are usually not affected by
oxidations
4. Substitution:
The conversion of an alcohol and a carboxylic acid to an
ester and water, in the presence of an acid catalyst.
The reaction between methanol and acetic acid to form
methyl acetate: CH3OH + CH3COOH CH3COOCH3 + H2O
Click to add speaker notes
ME
Substitution reactions in organic chemistry involve the
replacement of one atom or group of atoms with another
atom or group of atoms in a molecule.
1. Cleavage reactions:
Ethers can be cleaved by strong acids, such as HBr or HI, to form
alkyl halides and alcohols.
2. Williamson ether synthesis:
ETHERS
This is a method for the synthesis of ethers by the reaction of an
alkoxide ion with an alkyl halide.
3. Acid-catalyzed dehydration:
An ether is converted into an alkene by the elimination of a water
molecule under acidic conditions.
4. Oxidation:
Ethers can be oxidized with strong oxidizing agents, such as
peracids or chromium reagents, to form carbonyl compounds.
However, ethers are generally less prone to oxidation compared
to alcohols
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning