1. Determine the relationship between the two structures below. Circle your answer. H. Br H H CH3 H (CH3)3C-H CI-CI AH°-400 kJ/mol AH° 243 kJ/mol Br + (a) Enantiomers (b) Diastereomers 2. The bond dissociation energies for the relevant bonds are given below. Calculate the overall AHⓇ for the reaction. Place your answer in the box without units. (c) Identical (d) Constitutional isomers → (CH3)3C-CI + H-CI AH°-349 kJ/mol AH-432 kJ/mol kJ/mol
1. Determine the relationship between the two structures below. Circle your answer. H. Br H H CH3 H (CH3)3C-H CI-CI AH°-400 kJ/mol AH° 243 kJ/mol Br + (a) Enantiomers (b) Diastereomers 2. The bond dissociation energies for the relevant bonds are given below. Calculate the overall AHⓇ for the reaction. Place your answer in the box without units. (c) Identical (d) Constitutional isomers → (CH3)3C-CI + H-CI AH°-349 kJ/mol AH-432 kJ/mol kJ/mol
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter12: Chirality
Section: Chapter Questions
Problem 18E
Related questions
Question
Only typed explanation of both questions otherwise otherwise leave it
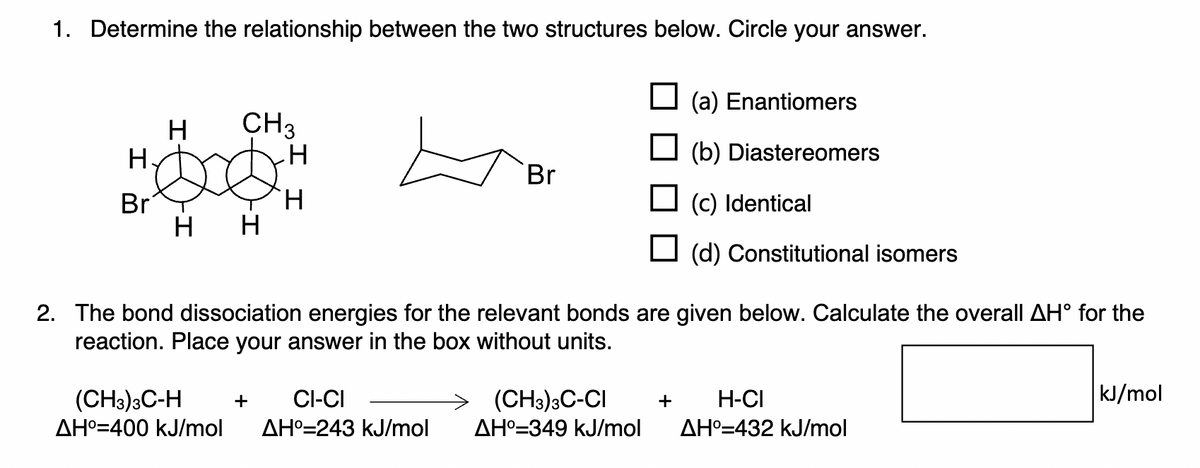
Transcribed Image Text:1. Determine the relationship between the two structures below. Circle your answer.
H
Br
H
H
CH3
H
H
H
+
(CH3)3C-H
CI-CI
AH⁰=400 kJ/mol AH°=243 kJ/mol
Br
(a) Enantiomers
(b) Diastereomers
2. The bond dissociation energies for the relevant bonds are given below. Calculate the overall AH° for the
reaction. Place your answer in the box without units.
(CH3)3C-CI
AH° 349 kJ/mol
☐ (c) Identical
(d) Constitutional isomers
+ H-CI
AH°=432 kJ/mol
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning