Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter21: Organic Chemistry
Section: Chapter Questions
Problem 23E: Is the general formula of a cycloalkanes the same as the general formula of an alkane, CnH2n+2? Draw...
Related questions
Question

Transcribed Image Text:YOUR ACETANILIDE RECOVERED IS: 2.945 GRAMS. MELTING POINT OF YOUR PRODUCT IS 234°C.
YOUR BENZIL RECOVERED IS 0.98 GRAMS. THE MELTING POINT OF YOUR BENZIL IS 201.9°C.
QUESTIONS TO BE ANSWERED IN THE REPORT:
1. GIVEN THE MELTING POINT OF YOUR ACETANILIDE PRODUCT, IS IT PURE OR IMPURE? EXPLAIN YOUR ANSWER BASED ON THE
MELTING POINT.
2. GIVEN THE MELTING POINT OF YOUR BENZIL PRODUCT, IS IT PURE OR IMPURE? EXPLAIN YOUR ANSWER BASED ON THE
MELTING POINT.
3. WHAT IS THE % YIELD OF ACETANILIDE? % YIELD OF BENZIL? SHOW YOUR CALCULATIONS.
4. IF I WERE TO MIX BENZIL AND ACETANILIDE TOGETHER, WOULD THERE BE A WAY TO DETERMINE THAT IT WAS A MIXTURE?
EXPLAIN HOW TO DETERMINE THIS.
5. IF PRODUCTS WERE NOT ALLOWED TO DRY, WOULD THAT AFFECT THE MELTING POINT? IN WHAT WAY COULD YOU DETERMINE
THE EFFECT?
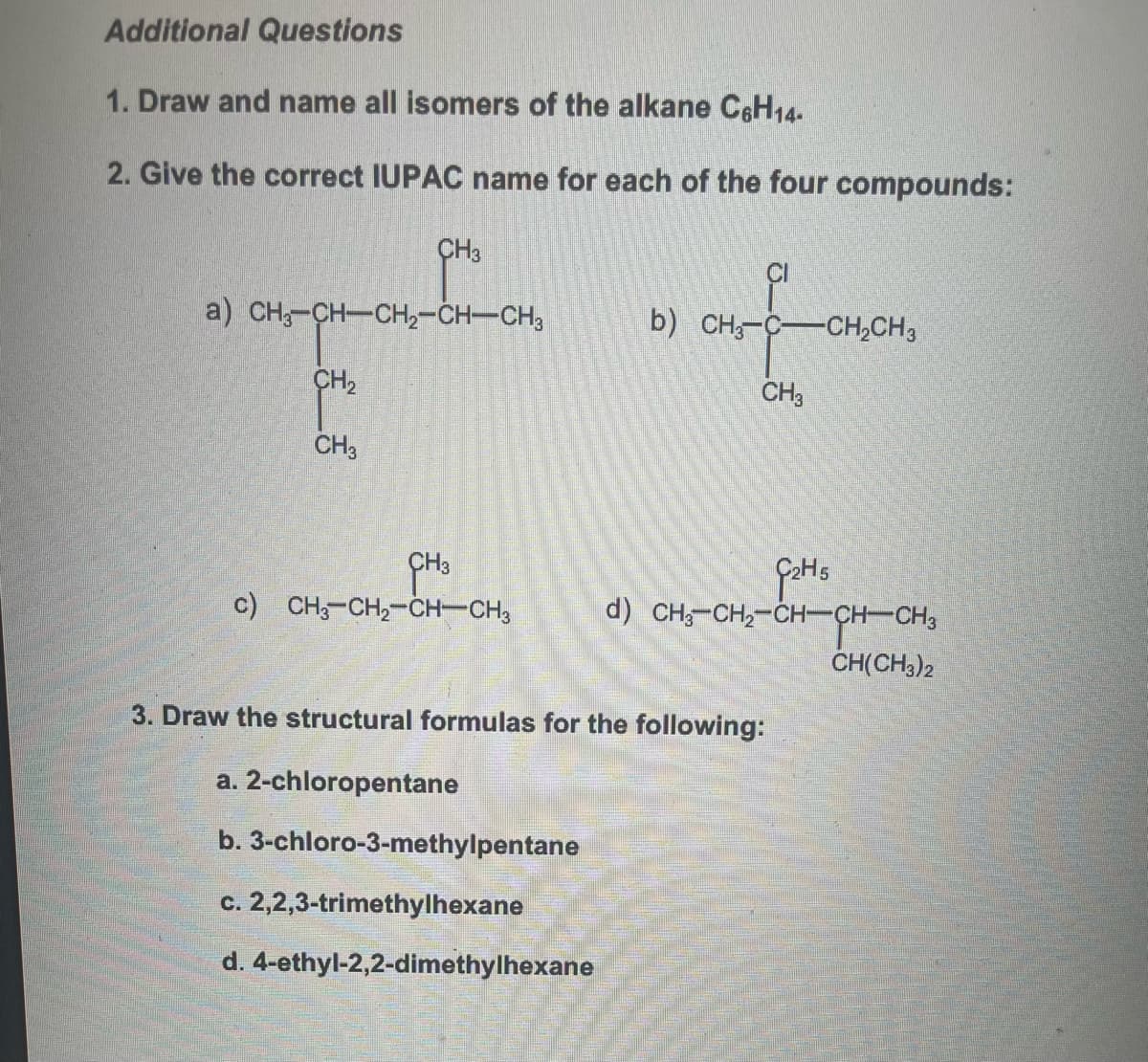
Transcribed Image Text:Additional Questions
1. Draw and name all isomers of the alkane C6H14-
2. Give the correct IUPAC name for each of the four compounds:
CH3
a) CH3-CH—CH,CHCH3
CH₂
CH3
c) CH3-CH₂-CH-CH3
CH3
c. 2,2,3-trimethylhexane
d. 4-ethyl-2,2-dimethylhexane
CI
b) CH3-C- -CH₂CH3
CH3
3. Draw the structural formulas for the following:
a. 2-chloropentane
b. 3-chloro-3-methylpentane
G₂H5
d) CHỖ CH,CHCHCH3
CH(CH3)2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co