Which of the following best describes the lone pairs on oxygen in the following aromatic molecule. The two lone pairs on oxygen are not shown in the picture below. O One lone pair on oxygen is in an sp² orbital and the other is an unhybridized p-orbital and participates in aromaticity. O Both lone pairs on oxygen are in an sp3 orbitals and can't participate in aromaticity. Both lone pairs on oxygen are in unhybridized p-orbitals and both participate in aromaticity. O The lone pairs of oxygen have nothing to do with this compound being aromatic.
Which of the following best describes the lone pairs on oxygen in the following aromatic molecule. The two lone pairs on oxygen are not shown in the picture below. O One lone pair on oxygen is in an sp² orbital and the other is an unhybridized p-orbital and participates in aromaticity. O Both lone pairs on oxygen are in an sp3 orbitals and can't participate in aromaticity. Both lone pairs on oxygen are in unhybridized p-orbitals and both participate in aromaticity. O The lone pairs of oxygen have nothing to do with this compound being aromatic.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.16P: Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hckel criteria?...
Related questions
Question
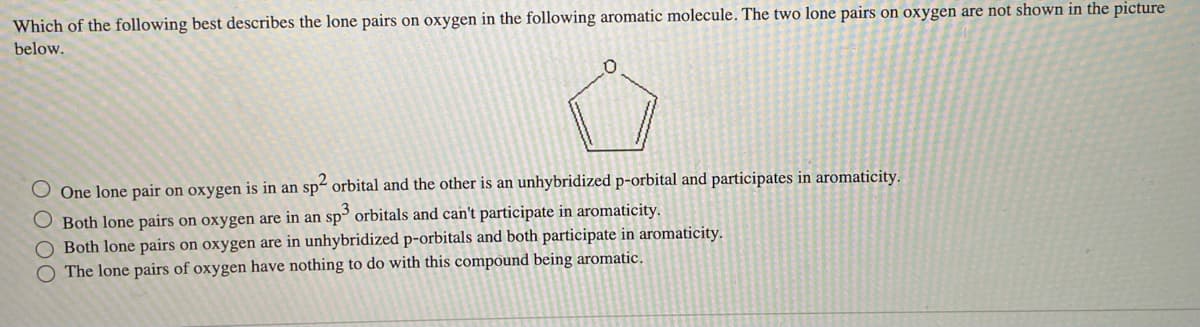
Transcribed Image Text:Which of the following best describes the lone pairs on oxygen in the following aromatic molecule. The two lone pairs on oxygen are not shown in the picture
below.
0
One lone pair on oxygen is in an sp² orbital and the other is an unhybridized p-orbital and participates in aromaticity.
O Both lone pairs on oxygen are in an sp3 orbitals and can't participate in aromaticity.
O Both lone pairs on oxygen are in unhybridized p-orbitals and both participate in aromaticity.
O The lone pairs of oxygen have nothing to do with this compound being aromatic.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning