1. Eighty grams of Silver was obtained from one hundred and forty grams of Silver nitrate. The Silver metal is prepared by reducing its nitrate. The chemical equation of the reaction is:
1. Eighty grams of Silver was obtained from one hundred and forty grams of Silver nitrate. The Silver metal is prepared by reducing its nitrate. The chemical equation of the reaction is:
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 103E: Silicon is produced for the chemical and electronics industries by the following reactions. Give the...
Related questions
Question
a. Balance the chemical equation;
b. Identify the limiting reactant and the excess reactant if applicable;
c. Compute for the theoretical yield;
d. Determine the percent yield of the reaction;
e. Calculate the percent error and
f. Compute for the excess amount of the excess reactant if applicable.
g. Show the complete solutions for your answers in a separate paper.
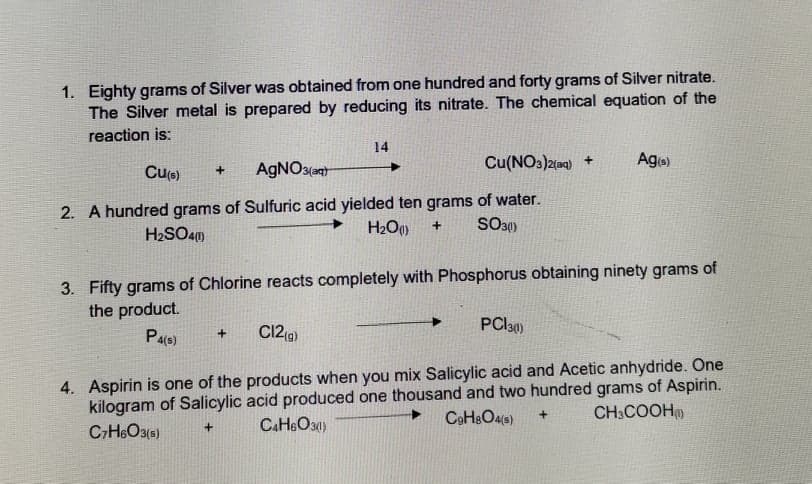
Transcribed Image Text:1. Eighty grams of Silver was obtained from one hundred and forty grams of Silver nitrate.
The Silver metal is prepared by reducing its nitrate. The chemical equation of the
reaction is:
14
Cu(NO3)2(aq) +
Age)
Cu(e)
AGNO3(
2. A hundred grams of Sulfuric acid yielded ten grams of water.
H2SO4)
H2O)
3. Fifty grams of Chlorine reacts completely with Phosphorus obtaining ninety grams of
the product.
Pa(s)
C12 (g)
PCI30)
4. Aspirin is one of the products when you mix Salicylic acid and Acetic anhydride. One
kilogram of Salicylic acid produced one thousand and two hundred grams of Aspirin.
CH6O3(s)
CoHsOas)
CH:COOH
CAH&O30)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning