Mass of AgCI (s) = Mole of AgCl = (mole = mass /molar mass) Calculate mole of AgNO, in the 10 mL unknown solution based on the following reaction equation: NaClag) + AGNO3(a0)NaNOyaq) + AgClo) Mole of AgNO, in the 10 mL unknown solution = use the coefficient ratio) _(hint, Mass of AGNO, in the 10 mL unknown solution = (mass = mole x molar mass) Calculate mass of AgNO, in the original unknown solution as follows: Mass of AGNO, in the original unknown solution volume of the unknown ARN03 solution in ml. 10 ml = Mass of AGNO, in the 10 mL unknown solution x
Mass of AgCI (s) = Mole of AgCl = (mole = mass /molar mass) Calculate mole of AgNO, in the 10 mL unknown solution based on the following reaction equation: NaClag) + AGNO3(a0)NaNOyaq) + AgClo) Mole of AgNO, in the 10 mL unknown solution = use the coefficient ratio) _(hint, Mass of AGNO, in the 10 mL unknown solution = (mass = mole x molar mass) Calculate mass of AgNO, in the original unknown solution as follows: Mass of AGNO, in the original unknown solution volume of the unknown ARN03 solution in ml. 10 ml = Mass of AGNO, in the 10 mL unknown solution x
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.7QAP
Related questions
Question
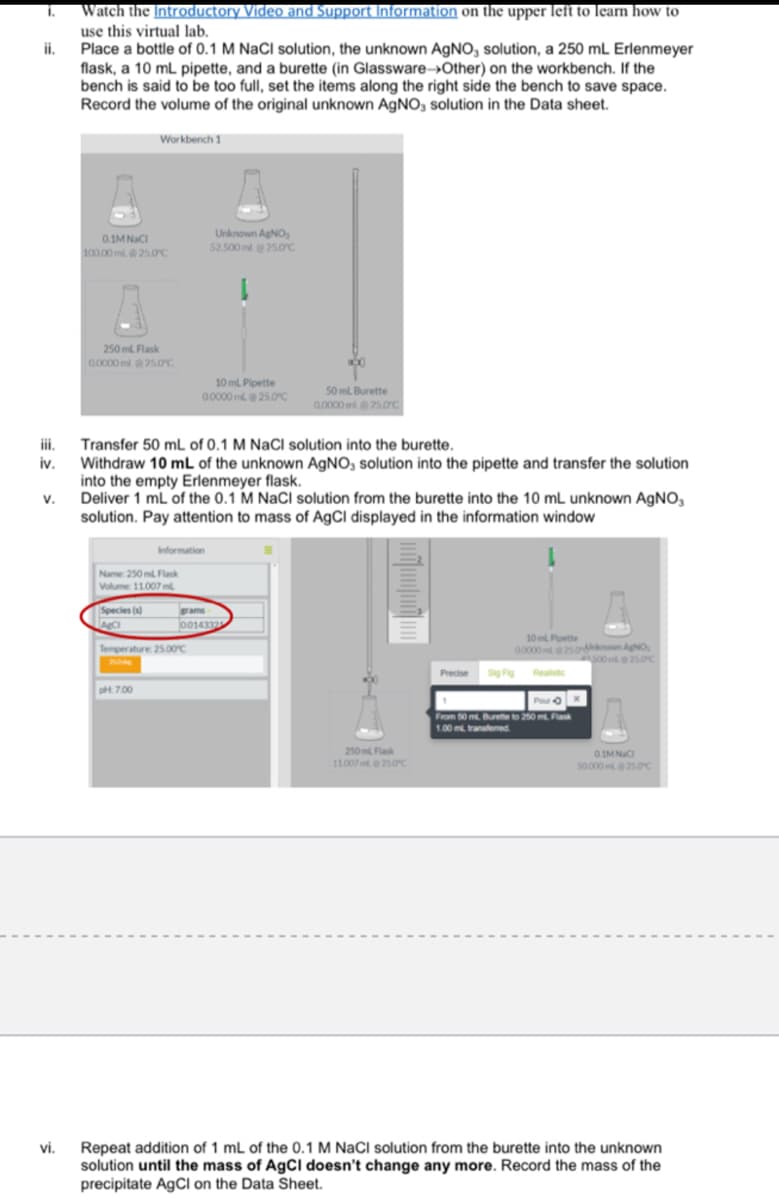
Transcribed Image Text:Watch the Introductory Video and Support Information on the upper left to learn how to
use this virtual lab.
i.
i.
Place a bottle of 0.1 M NaCl solution, the unknown AgNO, solution, a 250 mL Erlenmeyer
flask, a 10 mL pipette, and a burette (in Glassware→Other) on the workbench. If the
bench is said to be too full, set the items along the right side the bench to save space.
Record the volume of the original unknown AGNO, solution in the Data sheet.
Workbench 1
Unknown AgNO,
52.500 ml 25.0c
0.1MNaCI
100 00 ml 25.OC
250 ml Flask
0.0000 ml 25.0c
10 ml Pipette
00000 m25.0C
50 ml Burette
0.0000 ml25.0c
ii.
Transfer 50 mL of 0.1 M NaCl solution into the burette.
iv.
Withdraw 10 mL of the unknown AGNO, solution into the pipette and transfer the solution
into the empty Erlenmeyer flask.
v.
Deliver 1 mL of the 0.1 M NaCl solution from the burette into the 10 mL unknown AgNO,
solution. Pay attention to mass of AgCl displayed in the information window
Information
Name 250 m Flask
Volume 11.007 ml
Species (s)
ams
10 Poette
00000 5onown AgNO,
s0025.0C
Temperature 2500c
Precise
pt 700
From 50 m. Burete to 20 m Fa
1.00 m transtemed
250m Flak
11007250c
0IMNC
so000 250c
Repeat addition of 1 mL of the 0.1 M NaCl solution from the burette into the unknown
solution until the mass of AgCI doesn't change any more. Record the mass of the
precipitate AgCI on the Data Sheet.
vi.
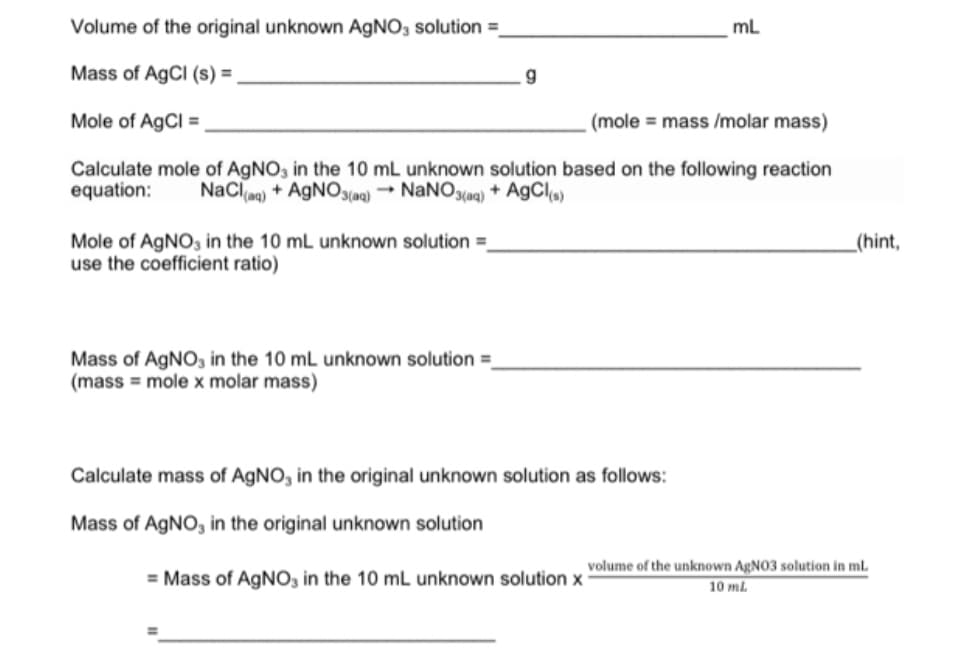
Transcribed Image Text:Volume of the original unknown AGNO, solution =
mL
Mass of AgCI (s) =
Mole of AgCl =
(mole = mass /molar mass)
Calculate mole of AgNO, in the 10 mL unknown solution based on the following reaction
equation:
NaCla)
+ AGNO3(aq) → NANO3(aq) + A9CI
Mole of AGNO, in the 10 mL unknown solution =
use the coefficient ratio)
_(hint,
Mass of AGNO, in the 10 mL unknown solution =
(mass = mole x molar mass)
Calculate mass of AGNO, in the original unknown solution as follows:
Mass of AGNO, in the original unknown solution
volume of the unknown AgNO3 solution in ml.
= Mass of AGNO, in the 10 mL unknown solution x
10 ml
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

