1. Ethylene (C2H4) is oxidized in a catalytic reactor to produce ethylene oxide (C2HẠO). At the same time, ethylene can also be combusted with oxygen to form carbon dioxide (CO2) and water (H2O). The reactions are demonstrated here: 2C,H, +0, →2C,H¸O C,H, +30, →2CO, + 2H,O Suppose a basis of calculation of 100 mol/s C2H4 entering the reactor and a separator is used to separate ethylene from CO2, H20, C2H4, and O2 perfectly. In another separator, CO2 and H2O are separated from C2H4 and O2. If there is 4 times more O2 present in the conduit entering the reactor than C2H4, 40 mol/s of C2H40 is produced and the selectivity of the ethylene oxide is 8 mol C2H4O/mol CO2. In addition, air is used to supply oxygen and the C2H4, O2, and N2 are recycled to the reactor inlet while using a purge. Suppose that 10% of the unused reagents (and N2) is purged and the rest is recycled to the reactor. Everything is shown in the diagram on the next page. (a) Do an analysis of the number of degrees of freedom for each subsystem, therefore: (1) Overall, (2) Mixing point, (3) Reactor, (4) Separator 1, (5) Separator 2, (6) Purge-recycling separation point (b) Explain why a purge is needed specifically in this process (c) Solve all the unknowns on the diagram (d) Determine the overall and single-pass conversions (in %) of C2H4
1. Ethylene (C2H4) is oxidized in a catalytic reactor to produce ethylene oxide (C2HẠO). At the same time, ethylene can also be combusted with oxygen to form carbon dioxide (CO2) and water (H2O). The reactions are demonstrated here: 2C,H, +0, →2C,H¸O C,H, +30, →2CO, + 2H,O Suppose a basis of calculation of 100 mol/s C2H4 entering the reactor and a separator is used to separate ethylene from CO2, H20, C2H4, and O2 perfectly. In another separator, CO2 and H2O are separated from C2H4 and O2. If there is 4 times more O2 present in the conduit entering the reactor than C2H4, 40 mol/s of C2H40 is produced and the selectivity of the ethylene oxide is 8 mol C2H4O/mol CO2. In addition, air is used to supply oxygen and the C2H4, O2, and N2 are recycled to the reactor inlet while using a purge. Suppose that 10% of the unused reagents (and N2) is purged and the rest is recycled to the reactor. Everything is shown in the diagram on the next page. (a) Do an analysis of the number of degrees of freedom for each subsystem, therefore: (1) Overall, (2) Mixing point, (3) Reactor, (4) Separator 1, (5) Separator 2, (6) Purge-recycling separation point (b) Explain why a purge is needed specifically in this process (c) Solve all the unknowns on the diagram (d) Determine the overall and single-pass conversions (in %) of C2H4
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Question Attached Below!
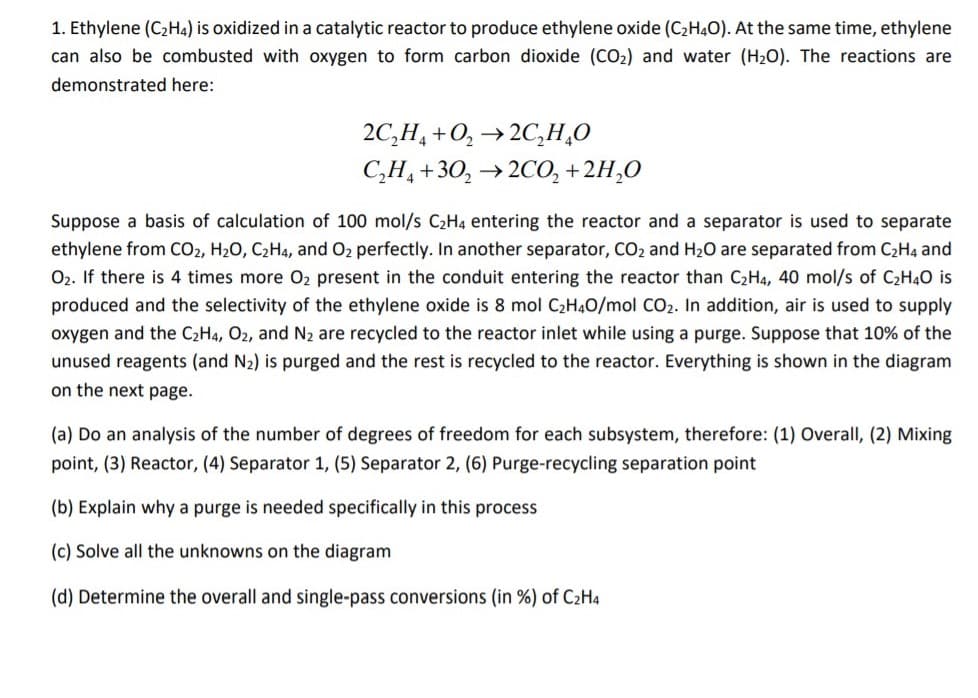
Transcribed Image Text:1. Ethylene (C2H4) is oxidized in a catalytic reactor to produce ethylene oxide (C2H4O). At the same time, ethylene
can also be combusted with oxygen to form carbon dioxide (CO2) and water (H20). The reactions are
demonstrated here:
2C,H, +0, →2C,H¸0
С,Н, +30, > 2СО, + 2H,0
Suppose a basis of calculation of 100 mol/s C2H4 entering the reactor and a separator is used to separate
ethylene from CO2, H20, C2H4, and O2 perfectly. In another separator, CO2 and H2O are separated from C2H4 and
02. If there is 4 times more 02 present in the conduit entering the reactor than C2H4, 40 mol/s of C2H40 is
produced and the selectivity of the ethylene oxide is 8 mol C2H40/mol CO2. In addition, air is used to supply
oxygen and the C2H4, O2, and N2 are recycled to the reactor inlet while using a purge. Suppose that 10% of the
unused reagents (and N2) is purged and the rest is recycled to the reactor. Everything is shown in the diagram
on the next page.
(a) Do an analysis of the number of degrees of freedom for each subsystem, therefore: (1) Overall, (2) Mixing
point, (3) Reactor, (4) Separator 1, (5) Separator 2, (6) Purge-recycling separation point
(b) Explain why a purge is needed specifically in this process
(c) Solve all the unknowns on the diagram
(d) Determine the overall and single-pass conversions (in %) of C2H4
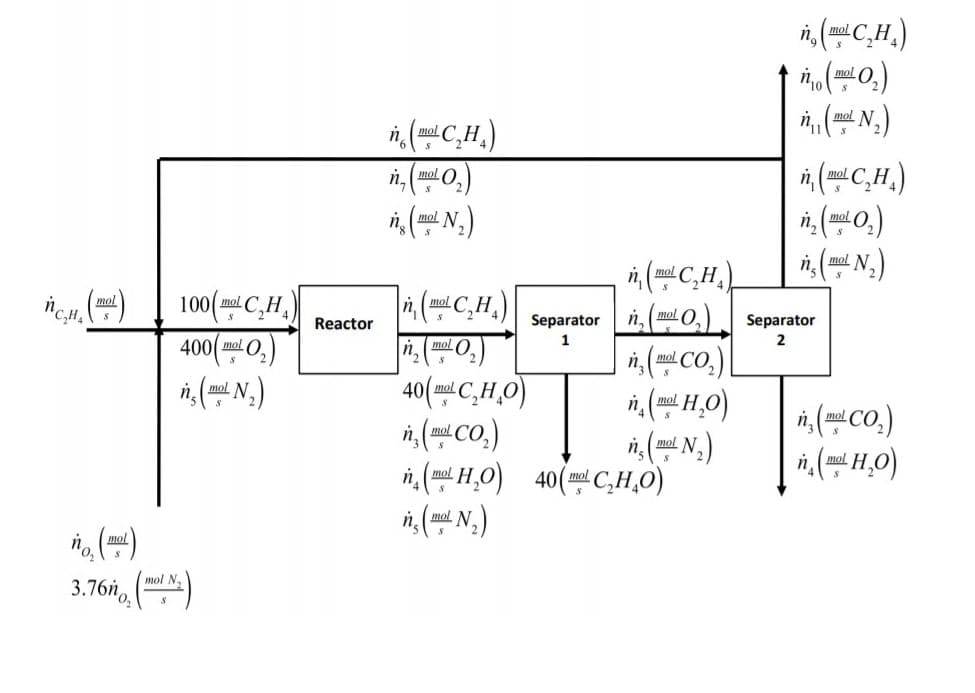
Transcribed Image Text:mol
(N.)
n, (0.)
mol
mol
mol N
i, (C,H,)
i, (C,H.)
100(ml C,H,
400(m 0,)
mol
mol C
Separator n, (mol O,
Separator
Reactor
n.mol O
i, (mal CO.)
40(m C,H,0)
n,( CO.)
n, (mil H,O) 40(mC,H,0)
n. mol N
i, (Co.)
mol N
()
3.76h, ()
mol N.
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The