Q: How many grams of silver nitrate must react to give 1.00 gg of AgAg?…
A:
Q: Each sketch below shows three objects with an electric charge. In each case, decide whether there is…
A: In general, two positively charged or negatively charged species repel each other and a positively…
Q: 19) Draw Products and label reaction mechanism 196) Why is this reaction inefficient at extending…
A: Gilman reagent.
Q: Complete the balanced dissociation equation for the compound below in aqueous solution. If the…
A:
Q: A student runs two experiments with a constant-volume "bomb" calorimeter containing 1500. g of water…
A: Given , Reaction : 2 C2H2(g) +5O2(g) →4CO2(g) +2H2O(g)
Q: D. IDENTIFYING A MESO COMPOUND. Which of the following structures are meso compounds? Encircle all…
A:
Q: (a) For each of the following structures A - D, indicate whether it is chiral or not. Explain. CI A…
A: A molecule is said to be chiral if it contains any asymmetric carbon atom that is the carbon in…
Q: For the combustion reaction of ethylene (C₂H4) C2H4 + 3O2 → 2 CO2 + 2 H₂O assume all reactants and…
A:
Q: A voltaic cell consists of a Pb/Pb2+Pb/Pb2+ half-cell and a Cu/Cu2+Cu/Cu2+ half-cell at 25 °C°C. The…
A:
Q: What is chemical incompatibility? What are the different types of chemical incompatibilities? Give…
A: 1) when the reaction occur between two or more substance and that reaction cause change in chemical…
Q: (b) 18.4 % C, 21.5 % N. and the rest Kans: KCN (c) 52.9 % Al, and the rest 0:0
A: Please go through my hand written notes for the solutions.
Q: Bromine monochloride is synthesized using the reaction Br₂(g) + Cl₂(g) = 2 BrCl(g) Kp = 1.1 x 10-4…
A: According to the question, The volume of the flask is given by = V = 208.0 L The mass of the Br2 is…
Q: At a certain temperature, the equilibrium constant, Ke, for this reaction is 53.3. H₂(g) + ₂(g) 2…
A:
Q: some metals form crusty oxide layers that can flake off to expose clean metal surfaces to moisture…
A: Given , Aluminium is more reactive than iron.
Q: How do you clean up a dilute acid spill?
A: We have been asked how do we clean up a dilute acid spill.Dilute acid is the acid in which acid is…
Q: Predict the major organic product(s) for each of the following reactions. Be sure to show…
A: m- CPBA stands for meta - chloroperbenzoic acid .It is used for epoxidation of alkene .It is an…
Q: The solubility product of Cu(OH)2 is 4.8 x 10-20. Calculate the value of pCu2+, i.e., -log[Cu2+], in…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: The vapor pressure of diethyl ether is 401 torr at 18 degrees C and it’s molar heat of vaporization…
A:
Q: Draw the mechanism for the reaction between 1-butene and HBr. Then, draw an energy diagram for the…
A: This is a addition reaction of HBr to the terminal alkene to give a secondery alkyl halide. This…
Q: What are the boiling point and freezing point of a 2.97 m solution of naphthalene in benzene?
A: we have to calculate the boiling point freezing point of 2.97 m solution of naphthalene in benzene…
Q: In the reaction of 1.11 g of salicylic acid (MM = 138.122 g/mol) with 2.89 g of acetic anhydride (MM…
A: Ans 1)
Q: One mole of a perfect gas expanded isothermally and reversibly at 0 °C from 1 to 1/10 bar. Calculate…
A: Given Initial pressure = 1 bar Final pressure= 0.1 bar Initial temperature= 0°C = 273 K
Q: Rank the following aqueous solutions in order of decreasing freezing point. Select 1 (highest…
A: Colligative properties: The colligative properties are those properties of solutions that depend on…
Q: Classify each of the following amines as primary, secondary or tertiary. CH3CH₂CH₂CH₂CH₂NH₂ NH₂…
A: We need to classify the given compounds to primary, secondary and tertiary amines. Amines are…
Q: Give specific examples of these chemical incompatibilities and their remedies 1.…
A: NOTE: Since you have asked for question containing several subparts, so we will answer only the…
Q: What is the IUPAC name of the following compound? CH3 0 CH3CHCH₂CCH3 Submit Answer Retry Entire…
A: Here structure of organic compound is given and we have to write down it's IUPAC nomenclature. To…
Q: What mass of ethylene glycol (C2H6O2, MM = 62.068 g/mol), in grams, would need to be used to prepare…
A: Given, Molarity of the solution = 12 M = 12 mol/L Volume of the solution = 125 mL = 0.125 L mass of…
Q: Which one of the reactions shown would produce (S)-3-methoxyheptane in high yield sodium…
A: Please go through my hand written notes for the detailed solution. Correct option is option (i)
Q: Determine the molecular formulas given the following empirical formulas and molar masses: bd (a)…
A: Molecular formula = (Empirical formula)n
Q: Determine the mass of deposited copper from the data in Table 8b-1
A: Mass of deposited copper has to be computed from the given initial mass of cathode and the final…
Q: Calculate the pH for each H₂O+ concentration. [H₂0+] = 1 x 10-6 M pH = pH = pH = [H₂0+] = 0.1 M…
A:
Q: In which of the following pure compounds would intermolecular hydrogen bonding be expected? (Select…
A: Given compound
Q: A compound with molecular formula C6H10 displays the following IR and Mass Spectrum. a) Calculate…
A: From IR spectrum.
Q: which one is the most suitable spectrum? (based on image) a/b/c/d/e
A: IR spectroscopy is mainly used for the identification of functional groups present in the molecules.…
Q: A voltaic cell is constructed in which the cathode is a standard hydrogen electrode and the anode is…
A:
Q: Draw the structure of each of the following molecules. (a) hexanedinitrile; (b)…
A:
Q: An unknown gas at 63.1 °C and 1.05 atm has a molar mass of 28.01 g/mol. Assuming ideal behavior,…
A: We are provided with an ideal gas of, T = 63.1 °C = 63.1+273.15 = 336.25K P = 1.05 atm m = 28.01…
Q: Part 2. Predict the Organic Products. Predict the major organic product(s) for each of the following…
A:
Q: Br₂ / H₂O "ОН Br Redraw the starting materials below and provide a reasonable mechanism for the…
A:
Q: C20H23CIN2O4 +2 HCIO4
A: The product is C20H24CLN2O4
Q: which one is the most suitable spectrum? (based on image) a/b/c/d/e
A: IR spectrum shows the characteristic stretching frequencies of various functional groups. It is…
Q: What is the name of the following? CI NO₂
A:
Q: Chemistry Draw the organic products obtained in each reaction. Hna IJNICE: 1221 131H₂O, A OE! [1]…
A: "Since you have asked multiple-subparts questions, we will solve the first three questions for you.…
Q: Question 21 of 21 What is the major product of the reaction shown below? H₂ Pt, heat A) butane B)…
A:
Q: Discuss the occurrence, isolation and properties of myricetin.
A: Myricetin is a polyphenolic compound having a molecular formula C15H10O8. The structure of a…
Q: What is the IUPAC name of each of the the following? 0 CH,CH, CHÍNH, CH₂CH3 0 CH3CH₂CHÖNHCH₂-…
A: In order to write IUPAC nomenclature we have to follow some standard rules of IUPAC 1) first select…
Q: Three moles of N₂(9), originally at 0.25 atm pressure, are mixed isothermally with 5 moles of H₂(g),…
A:
Q: 4 Br₂ CH₂Cl₂ OH Н+ ill ф Fill OH
A:
Q: Part 2. Predict the Organic Products. Predict the major organic product(s) for each of the following…
A:
Q: Four research teams measured the rotation period of a newly detected neutron star, and what each…
A: The measurement devices determine precision. The most precise equipment is a measurement device has…
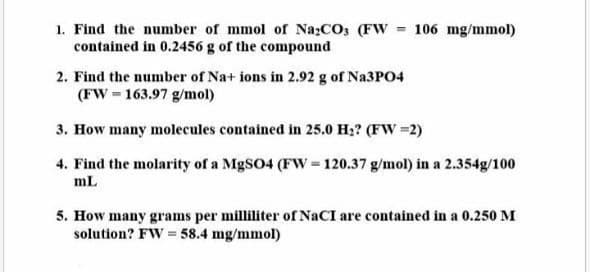
Step by step
Solved in 2 steps

- Hot perchloric acid is a powerful (and potentially explosive) reagent used to decompose organic materials and dissolve them for chemical analysis. (a) How many grams of perchloric acid, HClO4, are contained in 100.0 g of 70.5 wt% aqueous perchloric acid? (b) How many grams of water are in 100.0 g of solution? (c) How many moles of HClO4 are in 100.0 g of solution?Show your work: a. How many moles is 55 g of NaCl? 0.025 g of NaHCO3? b. How many grams is0.5 moles of NaCl? 2.11 moles of NaHCO3? c. Molarity is defined as the moles of solute in 1 Liter of solution. What is the molarity 0.50 mol Co(NO3)2 in 0.25 L of solution What is the molarity of68 g of NiCl2 in 0.15 L of solutionWine is approximately 12% ethanol (CH3CH2OH) by volume. Ethanol has a molar mass of 46.06 g/mol and a density 0.789 g/mL. How many moles of ethanol are present in a 750-mL bottle of wine?
- How many moles of calcium hydroxide are made from 5.3 moles of water? CaC2 + 2H20 ---> C2H2 + Ca(OH)2The active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin an expectorant drug used to assist the expectoration ('bringing up') of phlegm from the airways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© Chesty Forte Cough contains 4.00 g of Guaiphenesin (C10H14O4). If the recommended dose for an adult is 350 mg of Guaiphenesin. How many moles of Guaiphenesin does this equal? (answer to 2 significant figures)1.) 10g of an imaginary molecule X is dissolved to form a 500 mL solution. Calculate the concentration of the solution (Molarity) if the molecular weight of X is 45g/mol. 2)Calculate the mass percent of C in CH4 (you can use 1 mole of methane as the basis for yourcalculations) 3)An imaginary reaction takes place as below: X + Y2 → X3Y Balance the equation. If 10 moles of X and 10 moles of Y reacts how many moles of X3Y is produced?
- 50 mL of a 0.9 Ca3(PO4)2 solution is added to a beaker. How many moles of Ca3(PO4)2 were added? How many grams oF Ca3(PO4)2 were added?How many grams of NH3 can be produced by mixing 14 g of N2 with 6.0 g of H2? Given: N2 + 3 H2 ------> 2 NH3 a.) 20g b.) 17g c.) 34g d.) 51gA vitamin syrup for infants contains 27.44 mg of zinc sulfate monohydrate (ZnSO4.H2O) and 100.00 mg of ascorbic acid (C6H8O6)for every 5 ml. How many molecules of zinc sulfate monohydrate does a 5 ml of vitamin have?
- Formula for drug Liptor is C33 H35 Fn2 O5 Ph=7 look at the number of Hs assuming that number is the same as in grams calculate m/m% if you dissolve drug in 150 ml of water?35g how many moles of c present in 2.23 moles of the drug how many moles of your drug present in 50.0 grams how many molecules in 50.0 g calculate the H+. OH- and pOHSulfur trioxide dissolves in water, producing H2SO4. How much sulfuric acid can be produced from 12.7 mL of water and 22.3g of SO3? How much reagent excess is left over?An aqueous solution known as Ringer's lactate is administered intravenously to trauma victims suffering from blood loss or severe burns. The solution contains the chloride salts of sodium, potassium, and calcium and is also 5.00 mM in sodium lactate (NaC3H5O3). How many grams of sodium lactate are needed to prepare 12.5 liters of Ringer's lactate? _____ g

