1. For a reversible carnot cycle starting with an isothermal expansion with 2.50 mol of an ideal gas with a Cv = 5/2R as a working substance. The baths are at 298K and 825K, and Va = 2.25L Vb = 10.5L Vc = 134L Vd = 28.7L. a) What is the change in U, H, S, G, A for the whole cycle. b) What is the total work? Wt = Wab + Wbc + Wcd + Wda = Wab = Wbc = Wcd = Wda = c) What is the total heat? Wt = -qt= d) What is the efficiency? e = 1 - Tc/Th =
1. For a reversible carnot cycle starting with an isothermal expansion with 2.50 mol of an ideal gas with a Cv = 5/2R as a working substance. The baths are at 298K and 825K, and Va = 2.25L Vb = 10.5L Vc = 134L Vd = 28.7L. a) What is the change in U, H, S, G, A for the whole cycle. b) What is the total work? Wt = Wab + Wbc + Wcd + Wda = Wab = Wbc = Wcd = Wda = c) What is the total heat? Wt = -qt= d) What is the efficiency? e = 1 - Tc/Th =
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter18: Heat Engines, Entropy, And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 60P
Related questions
Question
100%
answer b and c please
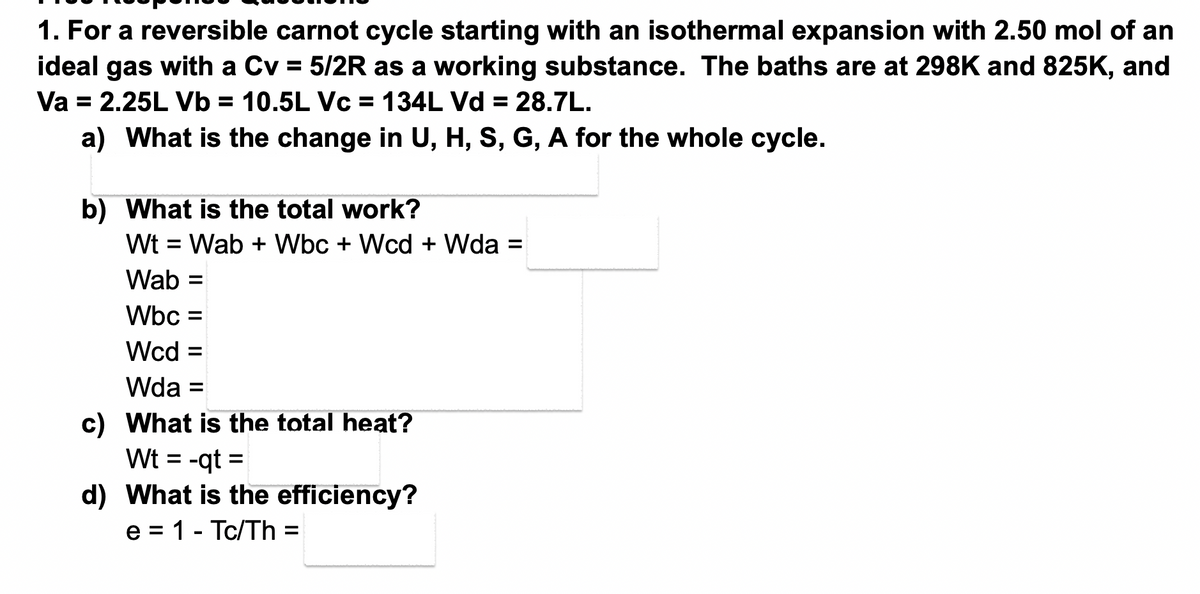
Transcribed Image Text:1. For a reversible carnot cycle starting with an isothermal expansion with 2.50 mol of an
ideal gas with a Cv = 5/2R as a working substance. The baths are at 298K and 825K, and
Va = 2.25L Vb = 10.5L Vc = 134L Vd = 28.7L.
a) What is the change in U, H, S, G, A for the whole cycle.
b) What is the total work?
Wt = Wab + Wbc + Wcd + Wda =
Wab =
Wbc =
Wcd =
Wda =
c) What is the total heat?
Wt = -qt=
d) What is the efficiency?
e = 1 - Tc/Th =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning