1. In both the starting material and product the chemical shift of the hydroxyl proton is approximately 13 ppm. Table 22.2 in your "Techniques in Organic Chemistry" textbook gives 4.0 - 8.0 ppm as the typical range for phenolic protons. Explain this apparent discrepancy. Hint: Think about the solvent the NMR spectrum was run in...
1. In both the starting material and product the chemical shift of the hydroxyl proton is approximately 13 ppm. Table 22.2 in your "Techniques in Organic Chemistry" textbook gives 4.0 - 8.0 ppm as the typical range for phenolic protons. Explain this apparent discrepancy. Hint: Think about the solvent the NMR spectrum was run in...
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL4: Proton (1h) Nmr Spectroscopy
Section: Chapter Questions
Problem 23CTQ
Related questions
Question
Transcribed Image Text:c2013902onno Part 5 •
Spectrometric Methods
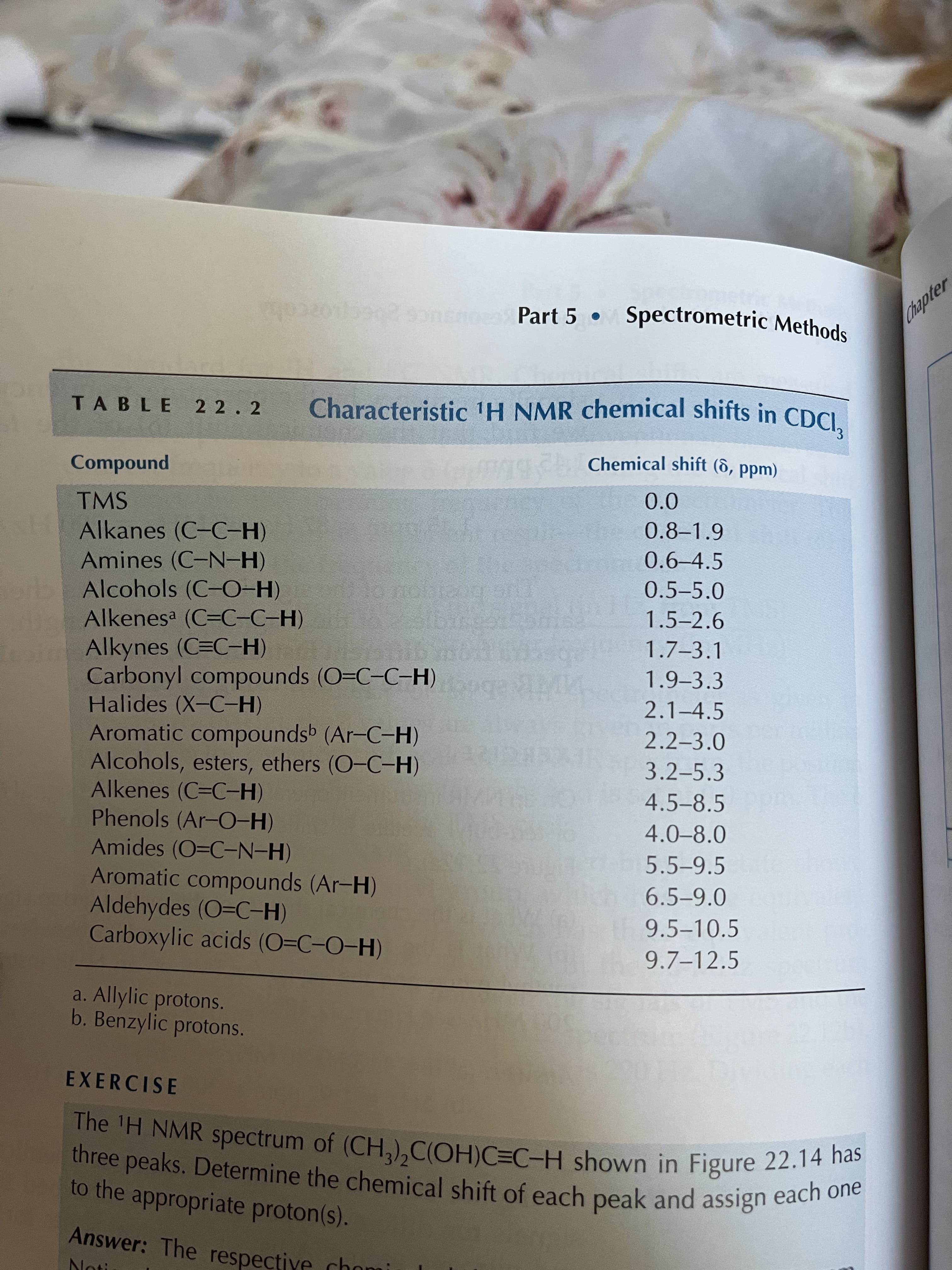
TABLE 22.2
Characteristic 'H NMR chemical shifts in CDCI.
Chemical shift (6, ppm)
punoduwoɔ
0.0
TMS
0.8-1.9
Alkanes (C-C-H)
0.6-4.5
Amines (C-N-H)
Alcohols (C-O-H)
Alkenesa (C=C-C-H)
Alkynes (C=C-H)
Carbonyl compounds (O=C-C-H)
Halides (X-C-H)
Aromatic compoundsb (Ar-C-H)
Alcohols, esters, ethers (O-C-H)
Alkenes (C=C-H)
Phenols (Ar-O-H)
Amides (O=C-N-H)
Aromatic compounds (Ar-H)
Aldehydes (O=C-H)
Carboxylic acids (O=C-O-H)
0.5-5.0
1.5-2.6
1.7-3.1
1.9-3.3
2.1-4.5
2.2-3.0
3.2-5.3
4.5-8.5
4.0-8.0
5.5-9.5
6.5-9.0
9.5-10.5
9.7-12.5
a. Allylic protons.
b. Benzylic protons.
EXERCISE
The 'H NMR spectrum of (CH,),C(OH)C=C-H shown in Figure 22. one
three peaks. Determine the chemical shift of each peak and assign each
to the appropriate proton(s).
Answer: The respective ch

Transcribed Image Text:1. In both the starting material and product the chemical shift of the hydroxyl proton is
approximately 13 ppm. Table 22.2 in your “Techniques in Organic Chemistry" textbook gives 4.0
- 8.0 ppm as the typical range for phenolic protons. Explain this apparent discrepancy. Hint: Think
about the solvent the NMR spectrum was run in.….
Expert Solution
Step 1
Electron withdrawing group and intramolecular hydrogen bond interaction make a proton deshielded and thus results higher chemical shift.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning