1. intermolecular or intramolecular? 2. intermolecular or intramolecular? 3. a) vaporization/ deposition/ condensation/ freezing/ melting/ sublimation b)released or absorbed? 4) a)vaporization/ deposition/ condensation/ freezing/ melting/ sublimation b) released or absorbed?
1. intermolecular or intramolecular? 2. intermolecular or intramolecular? 3. a) vaporization/ deposition/ condensation/ freezing/ melting/ sublimation b)released or absorbed? 4) a)vaporization/ deposition/ condensation/ freezing/ melting/ sublimation b) released or absorbed?
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 17E
Related questions
Question
1. intermolecular or intramolecular?
2. intermolecular or intramolecular?
3. a) vaporization/ deposition/ condensation/ freezing/ melting/ sublimation
b)released or absorbed?
4) a)vaporization/ deposition/ condensation/ freezing/ melting/ sublimation
b) released or absorbed?
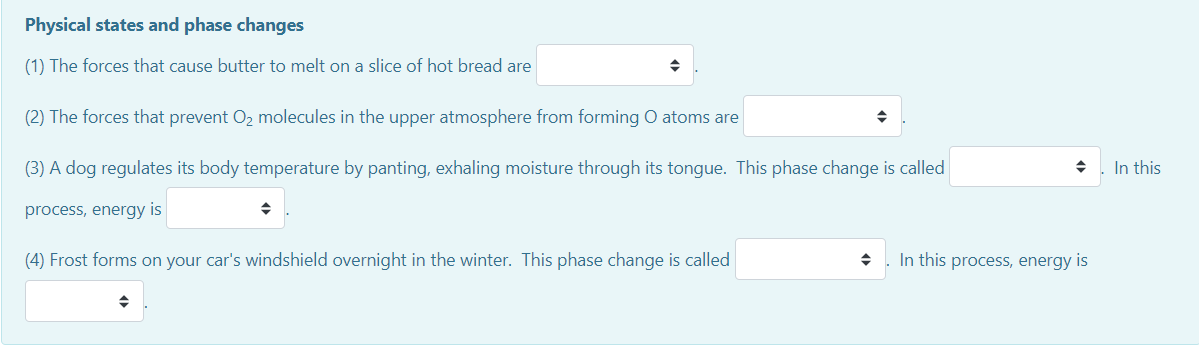
Transcribed Image Text:Physical states and phase changes
(1) The forces that cause butter to melt on a slice of hot bread are
(2) The forces that prevent O2 molecules in the upper atmosphere from forming O atoms are
(3) A dog regulates its body temperature by panting, exhaling moisture through its tongue. This phase change is called
In this
process, energy is
(4) Frost forms on your car's windshield overnight in the winter. This phase change is called
In this process, energy is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning



Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning