Stud Previous Page 27 of 36 E Next O References Use the References to access important values if needed for this question. Calculate the enthalpy of vaporization of C4H10- This compound has vapor pressures of 492 mmHg and 355 mmHg at -12.0 °C and -20.0 °C, respectively. (R = 8.314 J/mol-K) 20741Vemel USsigiment-take CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS Study Previous Page 28 of 36 E Next The normal boiling point of bromine is 58.8 °. Using the heat of vaporization of bromine (30.0 kJ/mol), calculate the vapor pressure of bromine at 14.5 °C. (R = 8.314 J/mol-K) 405 torr
Stud Previous Page 27 of 36 E Next O References Use the References to access important values if needed for this question. Calculate the enthalpy of vaporization of C4H10- This compound has vapor pressures of 492 mmHg and 355 mmHg at -12.0 °C and -20.0 °C, respectively. (R = 8.314 J/mol-K) 20741Vemel USsigiment-take CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS Study Previous Page 28 of 36 E Next The normal boiling point of bromine is 58.8 °. Using the heat of vaporization of bromine (30.0 kJ/mol), calculate the vapor pressure of bromine at 14.5 °C. (R = 8.314 J/mol-K) 405 torr
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 2QAP
Related questions
Question
Transcribed Image Text:Stud
Previous
Page 27 of 36 E
Next O
References
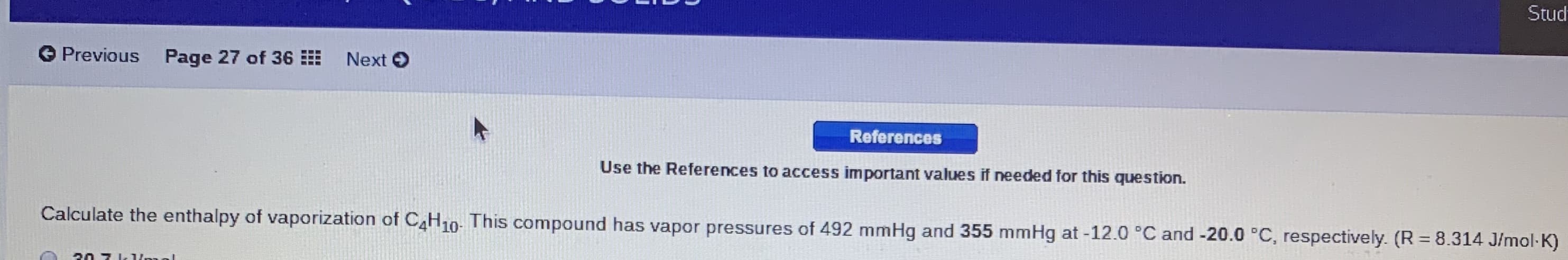
Use the References to access important values if needed for this question.
Calculate the enthalpy of vaporization of C4H10- This compound has vapor pressures of 492 mmHg and 355 mmHg at -12.0 °C and -20.0 °C, respectively. (R = 8.314 J/mol-K)
20741Vemel
Transcribed Image Text:USsigiment-take
CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS
Study
Previous Page 28 of 36 E Next
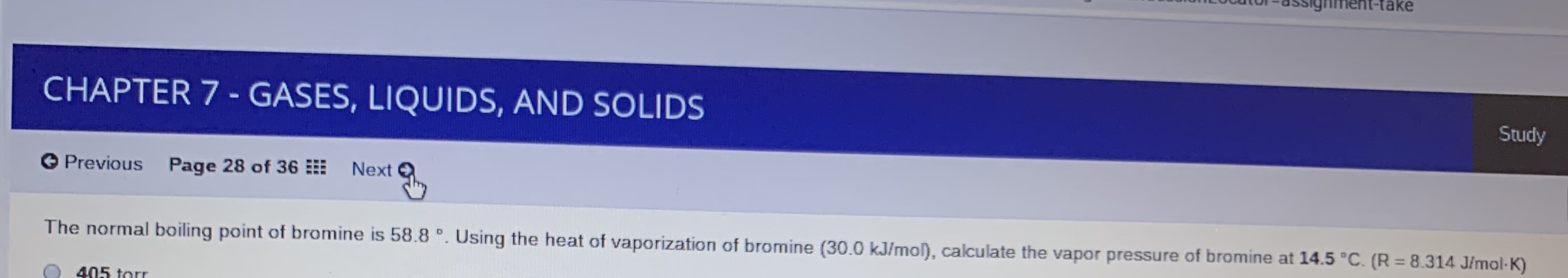
The normal boiling point of bromine is 58.8 °. Using the heat of vaporization of bromine (30.0 kJ/mol), calculate the vapor pressure of bromine at 14.5 °C. (R = 8.314 J/mol-K)
405 torr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax