1. List List three states of matter that are fluids. 2. Describe Complete the table below to describe four states of matter. State of matter Is shape definite or variable? Is volume definite or variable? Is it a fluid? How do the particles move? Are the particles electrically charged? Solid definite Liquid move past each other Gas no Plasma variable 3. Infer Which is easier to compress, a gas or a solid? Explain your answer. 4. Identify Relationships Which particles have more kinetic energy-those in a sub- stance with a high temperature or those in a substance with a low temperature?
1. List List three states of matter that are fluids. 2. Describe Complete the table below to describe four states of matter. State of matter Is shape definite or variable? Is volume definite or variable? Is it a fluid? How do the particles move? Are the particles electrically charged? Solid definite Liquid move past each other Gas no Plasma variable 3. Infer Which is easier to compress, a gas or a solid? Explain your answer. 4. Identify Relationships Which particles have more kinetic energy-those in a sub- stance with a high temperature or those in a substance with a low temperature?
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 86E
Related questions
Question
I need help with all five questions
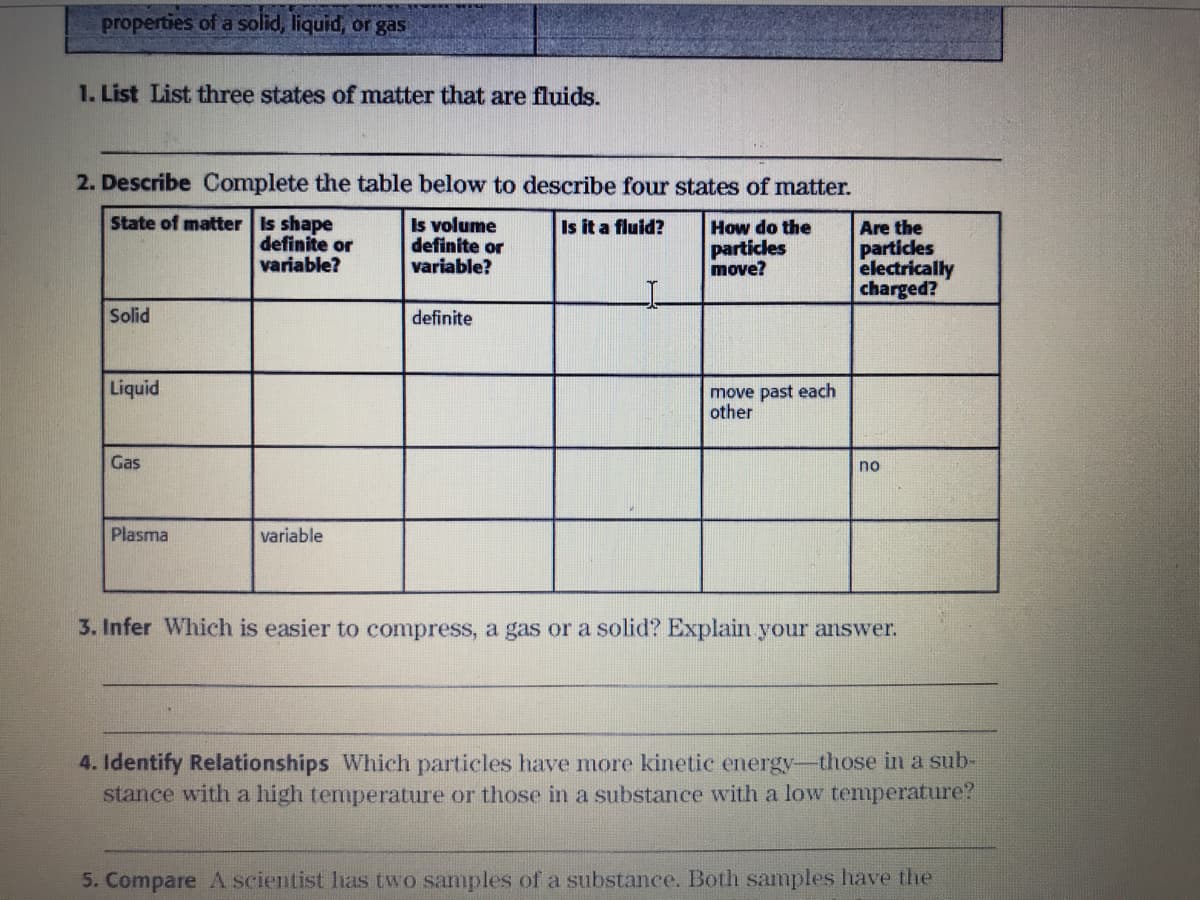
Transcribed Image Text:properties of a solid, liquid, or gas
1. List List three states of matter that are fluids.
2. Describe Complete the table below to describe four states of matter.
State of matter Is shape
definite or
variable?
Is volume
definite or
variable?
Is it a fluid?
Are the
particles
electrically
charged?
How do the
particles
move?
Solid
definite
Liquid
move past each
other
Gas
no
Plasma
variable
3. Infer Which is easier to compress, a gas or a solid? Explain your answer.
4. Identify Relationships Which particles have more kinetic energy-those in a sub-
stance with a high temperature or those in a substance with a low temperature?
5. Compare A scientist has two samples of a substance. Both samples have the
Transcribed Image Text:4. Identify Relationships Which particles have more kinetic energy-those in a sub-
stance with a high temperature or those in a substance with a low temperature?
5. Compare A scientist has two samples of a substance. Both samples have the
same temperature. One sample has a mass of 10 g. The other sample has a mass
of 20 g. Compare the average kinetic energy and total kinetic energy of the
particles in each sample.
Copyright O by Holt. Rinehart and Winston. All rights reserved.
Interactive Reader
48
States of Matter
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning