Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 137MP: A gaseous hydrocarbon reacts completely with oxygen gas to form carbon dioxide and water vapour....
Related questions
Question
Transcribed Image Text:Edit
View
History
Bookmarks
People
Tab
Window Help
w assignment x
8.7 Mole to Mol X
Edpuzzle
K Chemistry Refer x
classroom.google.com/u/0/c/MTYONTEXOTAYOTYW/a/MjgwNDc2NTEZMzA4/details
mmarly for Ch...
E Should College Be...
s DFS v7cjojo
s DFS v7 cj pro
Exit ticket
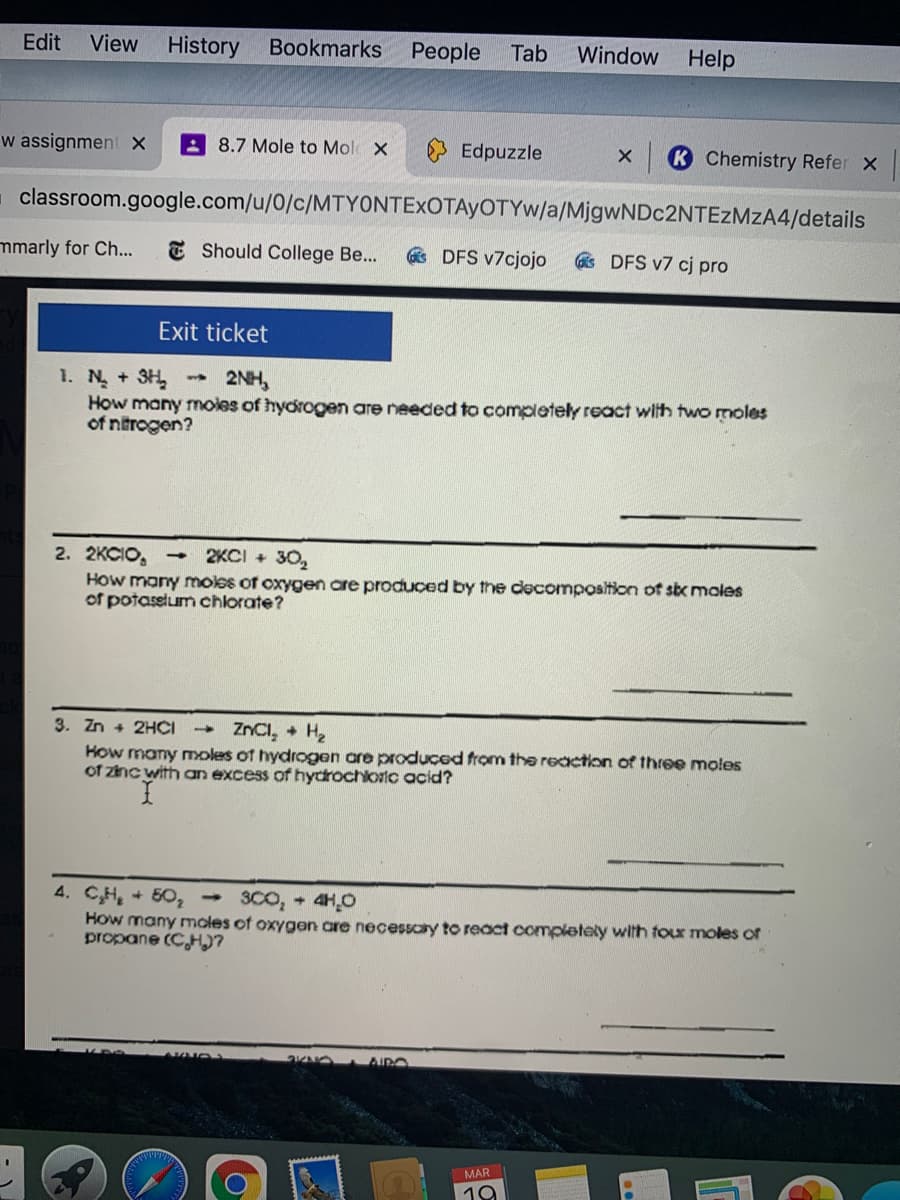
1. N +3H
How many moles of hydrogen are needed to completely react with two moles
of ntrogen?
2NH,
2KCI + 30,
2. 2KCIO, -
How many moles of oxygen are produced by the decomposition of six moles
of potassium chlorate?
ZNCI, + H
3. Zn + 2HCI
How many moles of hydrogen are produçed from the reaction of three moles
of znc with an excess of hydrochloric acid?
4. CH, + 50, 3C0, 4H,0
How many moles of oxygen are necessary to react completely with tour moles of
propane (CHI?
OKNO AIRO
MAR
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning