1. Nernst, Gibb's relationship to E° and equilibrium. Everything we've learned so far is inter-related. a. Fill out this concept map to link these things together mathematically: AG -EG (prods)-G(reacts) AG° = AH° -TAS AG -AG°: NON-STANDARD CONDITIONS STANDARD CONDITIONS E = K.K,,K,.K,.K.K, Keg 'cell EE, (ox)+E(red) b. What is the value of AG and Ecer at equilibrium? c. What are the signs of AG and Ecen when a reaction is spontaneous as written in the forward directic AG=-n FE
1. Nernst, Gibb's relationship to E° and equilibrium. Everything we've learned so far is inter-related. a. Fill out this concept map to link these things together mathematically: AG -EG (prods)-G(reacts) AG° = AH° -TAS AG -AG°: NON-STANDARD CONDITIONS STANDARD CONDITIONS E = K.K,,K,.K,.K.K, Keg 'cell EE, (ox)+E(red) b. What is the value of AG and Ecer at equilibrium? c. What are the signs of AG and Ecen when a reaction is spontaneous as written in the forward directic AG=-n FE
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.20QAP
Related questions
Question
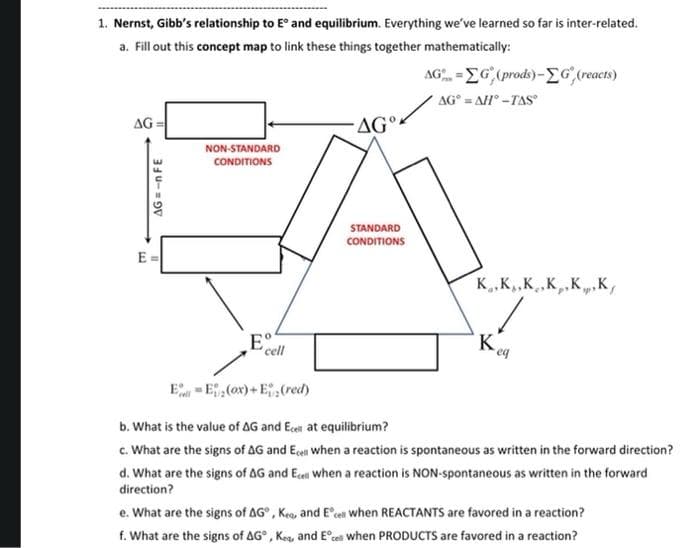
Transcribed Image Text:1. Nernst, Gibb's relationship to E° and equilibrium. Everything we've learned so far is inter-related.
a. Fill out this concept map to link these things together mathematically:
AG -EG, (prods)-G,(reacts)
AG° = AH° -TAS
AG =
AG°-
NON-STANDARD
CONDITIONS
STANDARD
CONDITIONS
E =
K.K,,K,.K,,K.K,
E
cell
'K
E-E,(ox) + E(red)
b. What is the value of AG and Ecen at equilibrium?
c. What are the signs of AG and Ecen when a reaction is spontaneous as written in the forward direction?
d. What are the signs of AG and Ecen when a reaction is NON-spontaneous as written in the forward
direction?
e. What are the signs of AG°, Keo, and Ecel when REACTANTS are favored in a reaction?
f. What are the signs of AG°, Keg, and E°ces when PRODUCTS are favored in a reaction?
AG =-n FE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning