1. Simple Cubic Unit Cell (b) Fig 5. Simple Cubic (SC) Unit Cell. (a) Ball-and-Stick Model (b) Space-filled Model (c) Stacking (a) (c) a. Each corner atom of the unit cell would be shared by how many unit cells? Answer: b. Assuming the atoms as perfect spheres, how much of a corner atom is used by just a single unit cell? Answer: c. If there are atoms at the eight corners of a simple cubic cell and each atom is shared by eight unit cells, how many total atoms are there per simple cubic unit cell? Answer:
1. Simple Cubic Unit Cell (b) Fig 5. Simple Cubic (SC) Unit Cell. (a) Ball-and-Stick Model (b) Space-filled Model (c) Stacking (a) (c) a. Each corner atom of the unit cell would be shared by how many unit cells? Answer: b. Assuming the atoms as perfect spheres, how much of a corner atom is used by just a single unit cell? Answer: c. If there are atoms at the eight corners of a simple cubic cell and each atom is shared by eight unit cells, how many total atoms are there per simple cubic unit cell? Answer:
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 5RQ: What is a lattice? What is a unit cell? Describe a simple cubic unit cell. How many net atoms are...
Related questions
Question
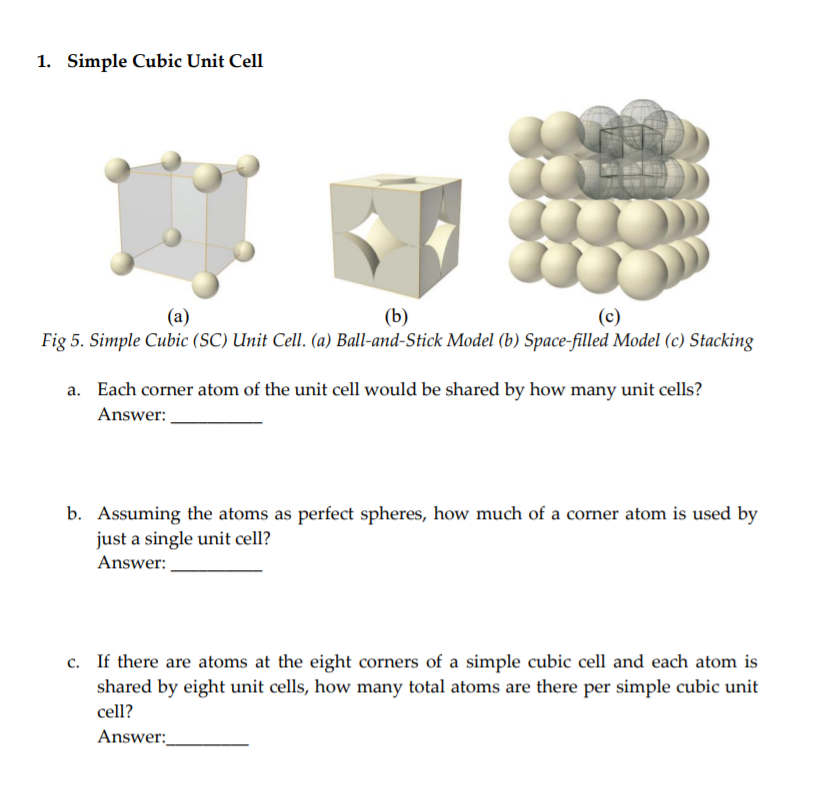
Transcribed Image Text:1. Simple Cubic Unit Cell
(b)
Fig 5. Simple Cubic (SC) Unit Cell. (a) Ball-and-Stick Model (b) Space-filled Model (c) Stacking
(a)
a. Each corner atom of the unit cell would be shared by how many unit cells?
Answer:
b. Assuming the atoms as perfect spheres, how much of a corner atom is used by
just a single unit cell?
Answer:
c. If there are atoms at the eight corners of a simple cubic cell and each atom is
shared by eight unit cells, how many total atoms are there per simple cubic unit
cell?
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning