Not all unit cells have a cubic structure. Take for example the below image wherein the unit cell takes the shape of a parallelepiped and has lengths a, b, c and angles a B. y. This particular unit cell has an atom arrangement where it has one atom at each corner, one central atom at each of the bases, one atom at the center of the unit cell, and one atom at the center of each of the edge lengths across length c. Given a density of 22.06 g/cm?, calculate the length of edge c in angstroms of a theoretical element Ro given the following unit cell dimensions in angstroms and degrees: a = 10.62A b = 6.1A y = 44° Atomic Mass: Ro: 52.406 g/mol
Not all unit cells have a cubic structure. Take for example the below image wherein the unit cell takes the shape of a parallelepiped and has lengths a, b, c and angles a B. y. This particular unit cell has an atom arrangement where it has one atom at each corner, one central atom at each of the bases, one atom at the center of the unit cell, and one atom at the center of each of the edge lengths across length c. Given a density of 22.06 g/cm?, calculate the length of edge c in angstroms of a theoretical element Ro given the following unit cell dimensions in angstroms and degrees: a = 10.62A b = 6.1A y = 44° Atomic Mass: Ro: 52.406 g/mol
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section9.6: Crystalline Solids
Problem 9.12E: Crystalline polonium has a primitive cubic unit cell, lithium has a body-centered cubic unit cell,...
Related questions
Question
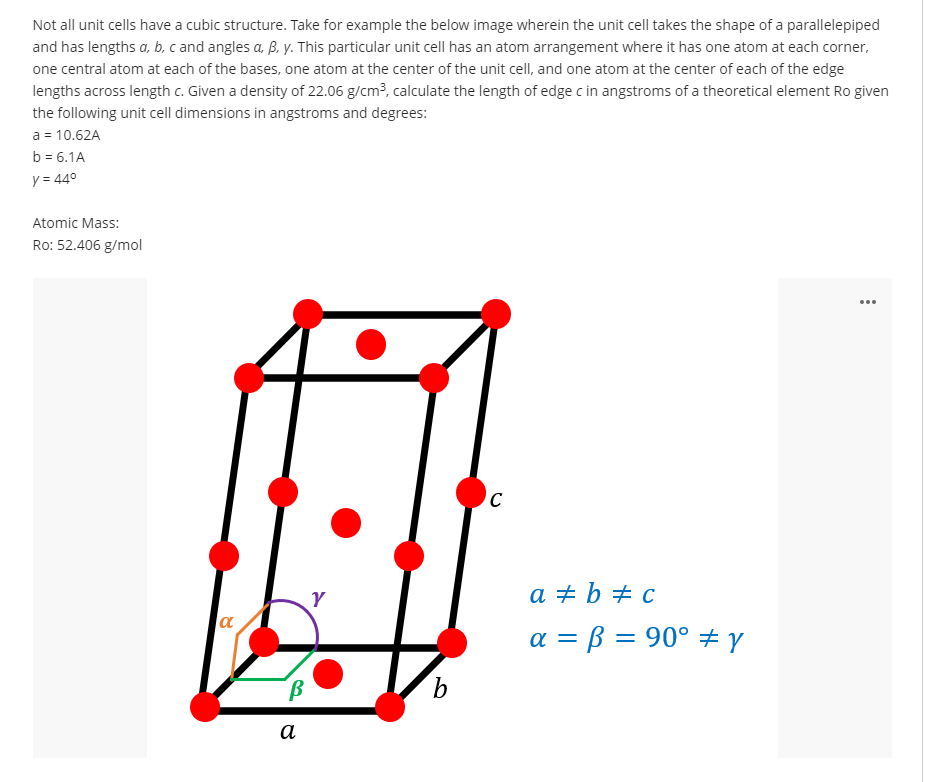
Transcribed Image Text:Not all unit cells have a cubic structure. Take for example the below image wherein the unit cell takes the shape of a parallelepiped
and has lengths a, b, c and angles a B, y. This particular unit cell has an atom arrangement where it has one atom at each corner,
one central atom at each of the bases, one atom at the center of the unit cell, and one atom at the center of each of the edge
lengths across length c. Given a density of 22.06 g/cm?, calculate the length of edge c in angstroms of a theoretical element Ro given
the following unit cell dimensions in angstroms and degrees:
a = 10.62A
b = 6.1A
y = 44°
Atomic Mass:
Ro: 52.406 g/mol
...
Y
a + b + c
a
a = B = 90° + y
9.
a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning