Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.4: Heat Versus Temperature: Heat Transfer
Problem 1TAI
Related questions
Question
Can you please solve question 1 on the attached document; showing step by step solution.
Thank you
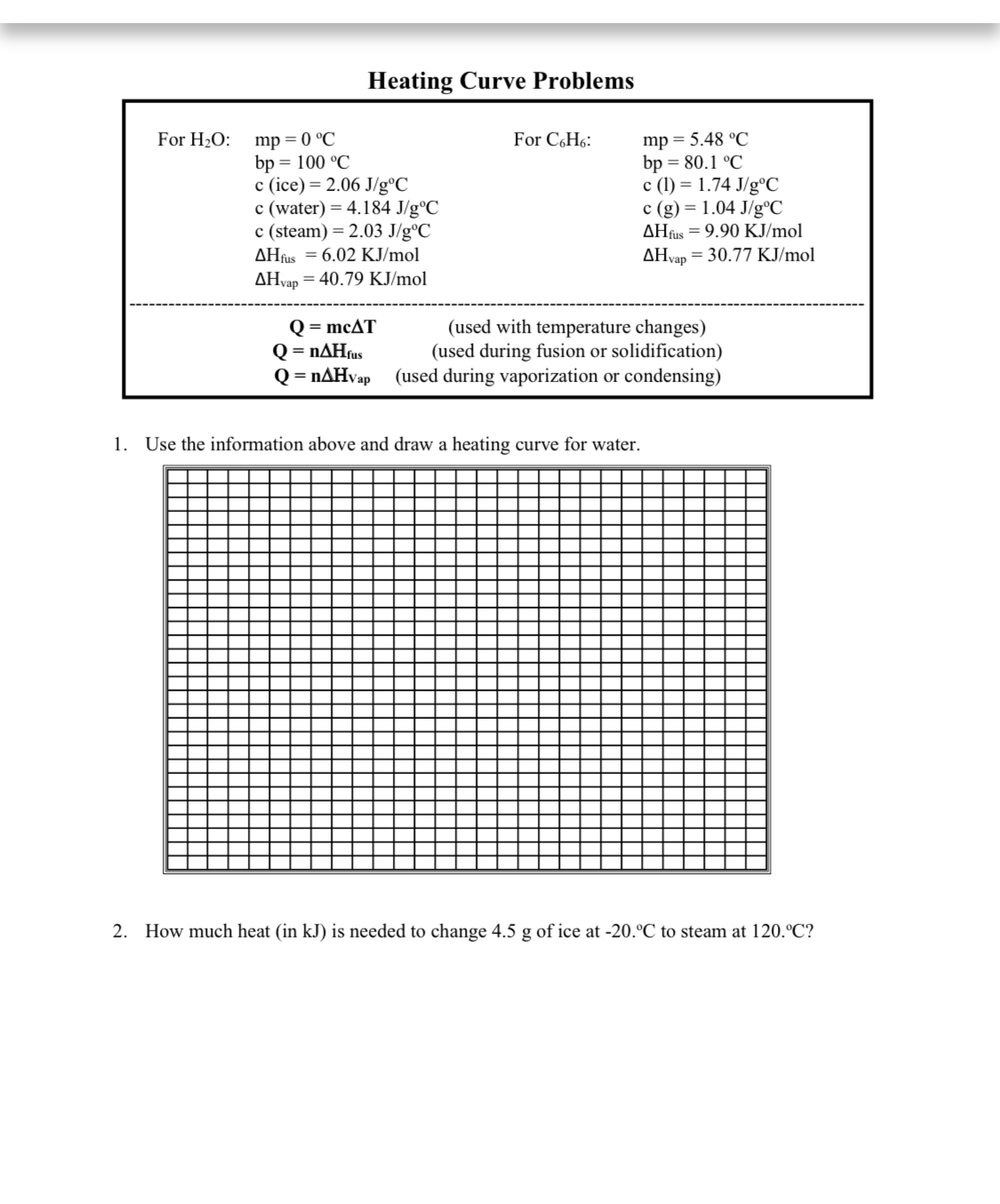
Transcribed Image Text:Heating Curve Problems
mp = 0 °C
bp = 100 °C
c (ice) = 2.06 J/g°C
c (water) = 4.184 J/g°C
c (steam) = 2.03 J/g°C
AHfus = 6.02 KJ/mol
mp = 5.48 °C
bp = 80.1 °C
c (1) = 1.74 J/g°C
c (g) = 1.04 J/g°C
AHfus = 9.90 KJ/mol
For H2O:
For C,H6:
AHvap = 30.77 KJ/mol
AHvap = 40.79 KJ/mol
Q = mcAT
Q = nAHfus
Q = nAHva
(used with temperature changes)
(used during fusion or solidification)
(used during vaporization or condensing)
Vap
1. Use the information above and draw a heating curve for water.
2. How much heat (in kJ) is needed to change 4.5 g of ice at -20.°C to steam at 120.°C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning