1. What evidence observed in this lab could be used to support the theory that electrons are found in definite energy levels around the nucleus?
1. What evidence observed in this lab could be used to support the theory that electrons are found in definite energy levels around the nucleus?
Biology Today and Tomorrow without Physiology (MindTap Course List)
5th Edition
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cecie Starr, Christine Evers, Lisa Starr
Chapter2: Molecules Of Life
Section: Chapter Questions
Problem 3CT: Polonium is a rare element with 33 radioisotopes. The most common one, 210Po, has 82 protons and 128...
Related questions
Question
100%
Do the first question please
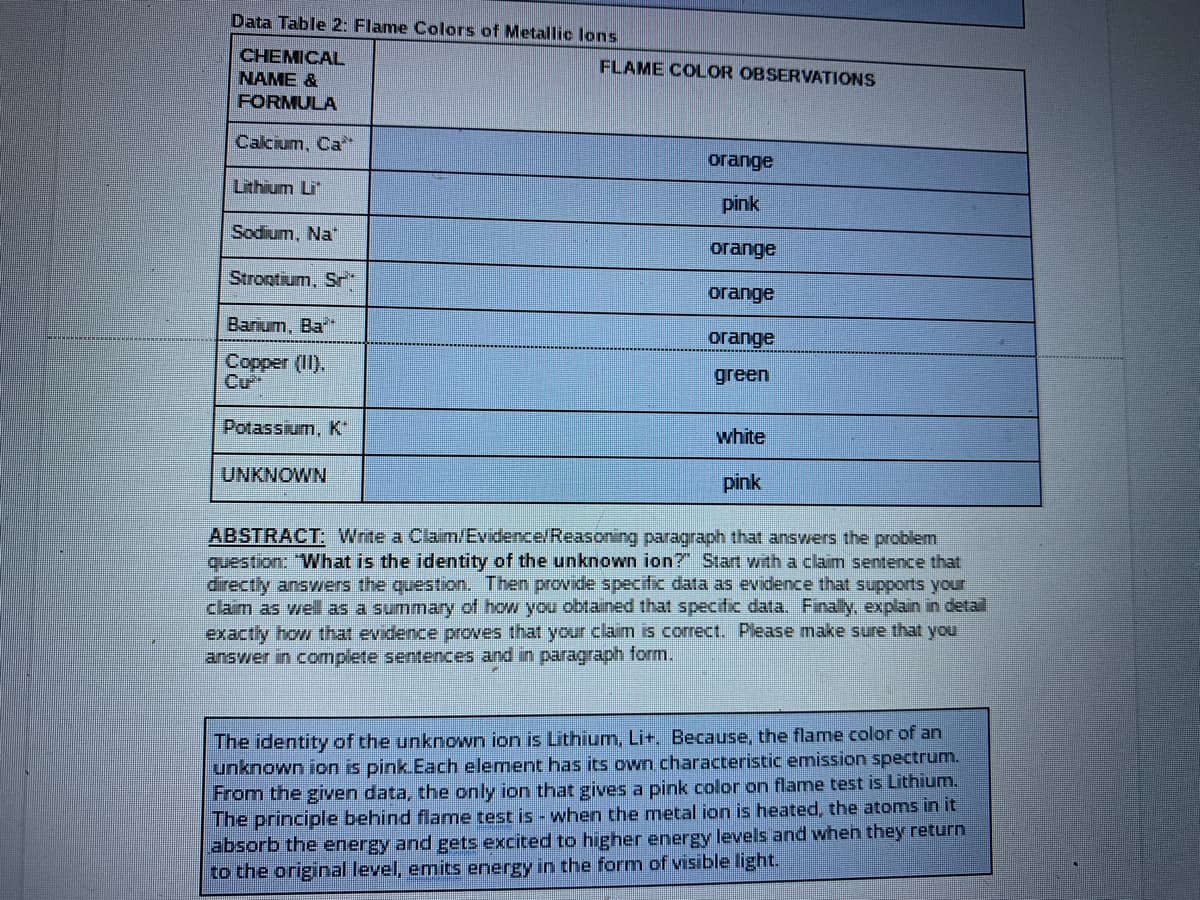
Transcribed Image Text:Data Table 2: Flame Colors of Metallic lons
CHEMICAL
NAME &
FLAME COLOR OBSERVATIONS
FORMULA
Calcium, Ca
orange
Lithium Li
pink
Sodium, Na"
orange
Strootium, Sr*
огаnge
Barium, Ba*
orange
Copper (II).
Cư
green
Potassium, K
white
UNKNOWN
pink
ABSTRACT: Wnte a Claim/Evidence/Reasoning paragraph that answers the problem
question: "What is the identity of the unknown ion? Start with a claim sentence that
directly answers the question. Then provide specific data as evidence that supports your
claim as well as a summary of how you obtained that specific data. Finally, explain in detail
exactly how that evidence proves that your claim is correct. Please make sure that you
answer in complete sentences and in paragraph form.
The identity of the unknown ion is Lithium, Lit. Because, the flame color of an
unknown ion is pink Each element has its own characteristic emission spectrum.
From the given data, the only ion that gives a pink color on flame test is Lithium.
The principle behind flame test is - when the metal ion is heated, the atoms in it
absorb the energy and gets excited to higher energy levels and when they return
to the original level, emits energy in the form of visible light.
Transcribed Image Text:unknown ion is pink.Each element has its own characteristic emission spectrum.
From the given data, the only ion that gives a pink color on flame test is Lithium.
The principle behind flame test is - when the metal ion is heated, the atoms in it
absorb the energy and gets excited to higher energy levels and when they return
to the original level, emits energy in the form of visible light.
CONCLUSIONS/REFLECTIONS: Please answer the following question in the blue box
provided.
1. What evidence observed in this lab
could be used to support the theory that
electrons are found in definite energy levels
around the nucleus?
2. Explain how light is emitted when an
element gets excited.
3. How might emission spectra be used in
studying stars?
4. Why do you suppose that larger
elements such as neon produce more color
bands (1.e. line spectra) than smaller
elements ke hydrogen?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning


Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning



Principles Of Radiographic Imaging: An Art And A …
Health & Nutrition
ISBN:
9781337711067
Author:
Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:
Cengage Learning