This is a plot under standard conditions of free energy corresponding to a chemical reaction of A+B to C+D. What will happen if only C+D are present under standard temperature and pressure?
This is a plot under standard conditions of free energy corresponding to a chemical reaction of A+B to C+D. What will happen if only C+D are present under standard temperature and pressure?
Chapter7: Muscular System
Section: Chapter Questions
Problem 1CAC
Related questions
Question
This is a plot under standard conditions of free energy corresponding to a
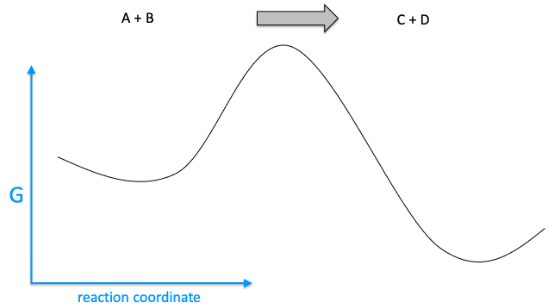
Transcribed Image Text:G
A + B
reaction coordinate
C+D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you





