1. When a solution of MgCl, is mixed with a solution of AGNO, a white precipitate forms. Balanced equation Total ionic equation Net ionic equation If aluminum metal, Al, is placed in a solution of Ni(NO),, it dissolves and a new 2. solid forms. Balanced equation Total ionic equation Net ionic equation 3. A white precipitate forms when solutions of lithium sulfate, Li,SO,, and calcium chloride, CaCI, are combined. Balanced equation Total ionic equation Net ionic equation 4. Zinc metal, Zn, reacts with a solution of HCI to form hydrogen gas, H,. Balanced equation Total ionic equation Net ionic equation
1. When a solution of MgCl, is mixed with a solution of AGNO, a white precipitate forms. Balanced equation Total ionic equation Net ionic equation If aluminum metal, Al, is placed in a solution of Ni(NO),, it dissolves and a new 2. solid forms. Balanced equation Total ionic equation Net ionic equation 3. A white precipitate forms when solutions of lithium sulfate, Li,SO,, and calcium chloride, CaCI, are combined. Balanced equation Total ionic equation Net ionic equation 4. Zinc metal, Zn, reacts with a solution of HCI to form hydrogen gas, H,. Balanced equation Total ionic equation Net ionic equation
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter17: Solubility And Complex-ion Equilibria
Section: Chapter Questions
Problem 17.15QP: Solubility and Solubility Product You put 0.10-mol samples of KNO3, (NH4)2S, K2S, MnS, AgCl, and...
Related questions
Question
100%
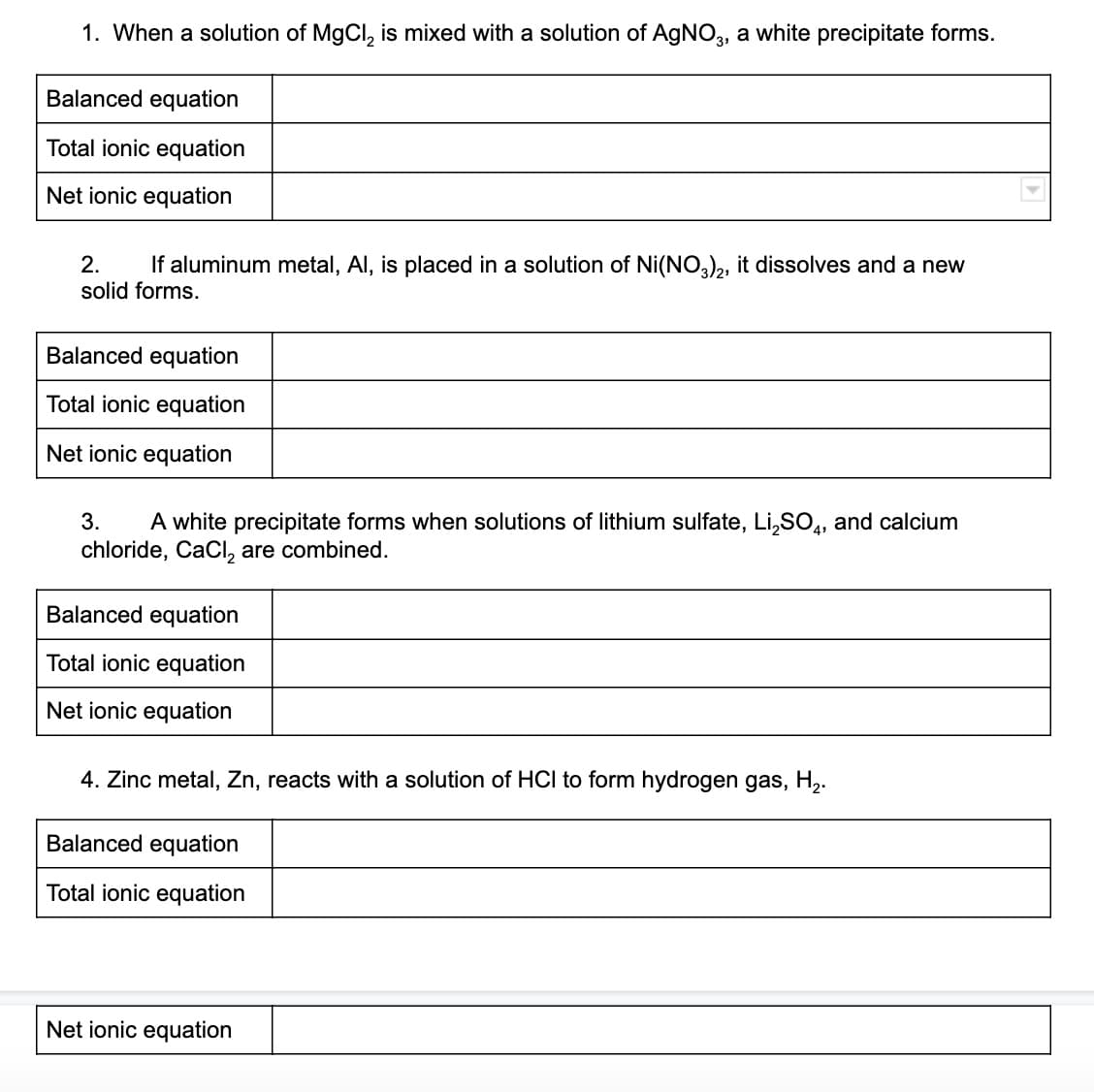
Transcribed Image Text:1. When a solution of MgCl, is mixed with a solution of AgNO, a white precipitate forms.
Balanced equation
Total ionic equation
Net ionic equation
2.
If aluminum metal, Al, is placed in a solution of Ni(NO), it dissolves and a new
solid forms.
Balanced equation
Total ionic equation
Net ionic equation
3.
A white precipitate forms when solutions of lithium sulfate, Li,SO,, and calcium
chloride, CaCI, are combined.
Balanced equation
Total ionic equation
Net ionic equation
4. Zinc metal, Zn, reacts with a solution of HCI to form hydrogen gas, H,.
Balanced equation
Total ionic equation
Net ionic equation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning