Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter4: Chemical Reactions In Solution
Section: Chapter Questions
Problem 4.9QE: Addition of water to concentrated sulfuric acid is dangerous because it generates enough heat to...
Related questions
Question
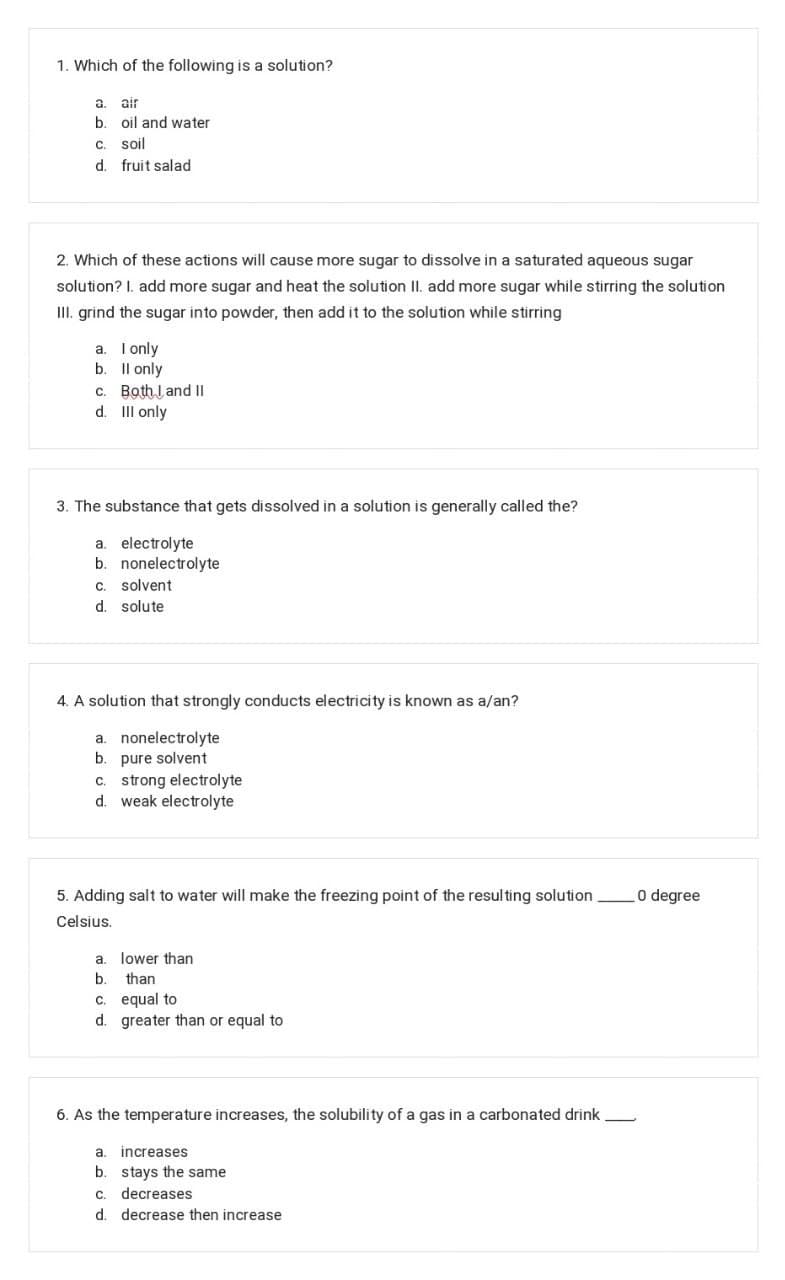
Transcribed Image Text:1. Which of the following is a solution?
a.
air
b.
oil and water
C
soil
d. fruit salad
2. Which of these actions will cause more sugar to dissolve in a saturated aqueous sugar
solution? I. add more sugar and heat the solution II. add more sugar while stirring the solution
III. grind the sugar into powder, then add it to the solution while stirring
a. I only
b. Il only
c. Both l and II
d. III only
3. The substance that gets dissolved in a solution is generally called the?
a. electrolyte
b. nonelectrolyte
c. solvent
d. solute
4. A solution that strongly conducts electricity is known as a/an?
a. nonelectrolyte
b. pure solvent
c. strong electrolyte
d. weak electrolyte
5. Adding salt to water will make the freezing point of the resulting solution
O degree
Celsius.
a. lower than
b.
than
c. equal to
d. greater than or equal to
6. As the temperature increases, the solubility of a gas in a carbonated drink
a.
increases
b. stays the same
C.
decreases
d. decrease then increase
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning