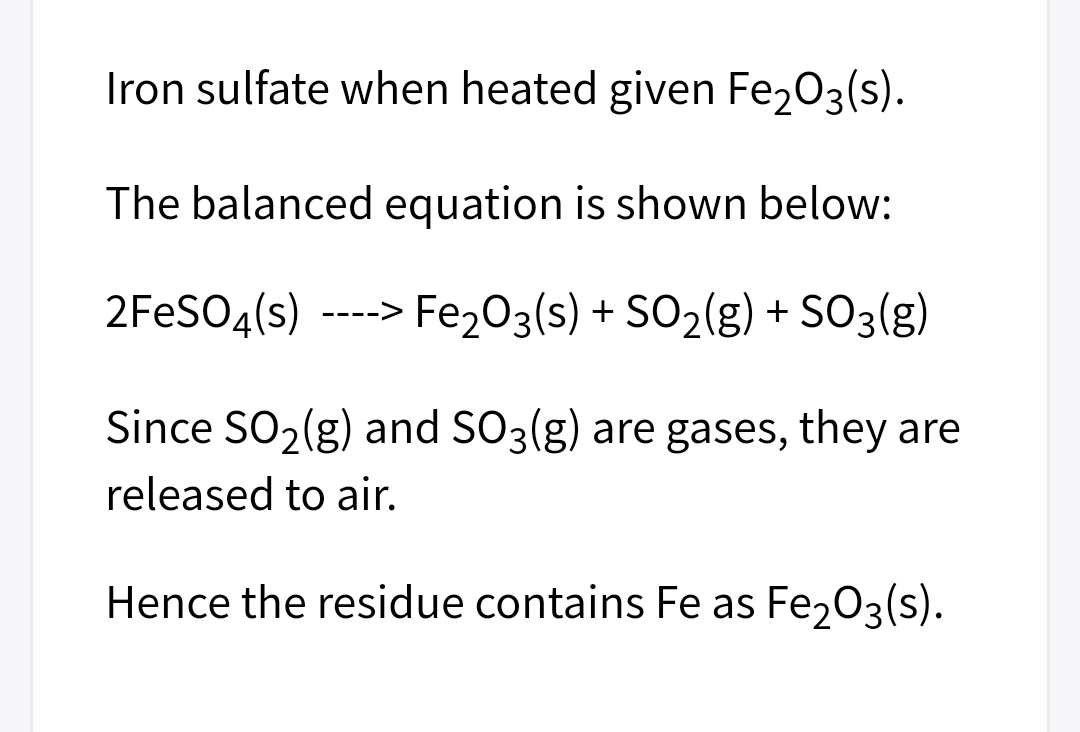
1. Write a balanced chemical reaction for the precipitation of Fe (after the addition of ammonium nitrate). 2. Why is AgNO3 used to test the presence of Cl? Write the chemical reaction.
Q: he alkalinity of the unknown groundwaters A and B has been acquired by the dissolution of NaHCO3 and…
A: Processes that inhibit pH changes in an aquifer are called pH buffering processes. These processes…
Q: Calculate the concentration (M) of sodium ions in a solution made by diluting 50.0 mL of a 0.874 M…
A: Given values: Initial volume: V1 = 50 mL Initial concentration: C1= 0.874 mL Final volume: V2 = 250…
Q: Draw the structure of EDTA^4- and explain why EDTA^2- can act as a buffer?
A: The compounds given are EDTA4- and EDTA2-.
Q: Is phosphate and phosphoric acid the same thing? Or are they different?
A: If phosphate and phosphoric acid the same thing or different is to be explained.
Q: If the Haber process favors the left side of the reaction in terms of equilibrium, how can you…
A: In Haber process, formation of NH3 takes place following the chemical equation: N2(g) + 3H2(g)…
Q: 5. Detenmine the moles and number of grams of calcium carbonate needed to make the following…
A: Given (a)25.0ml of 0.997M CaCO3 (b) 1.25L of 3.42M CaCO3 To find moles and mass of CaCO3
Q: Write chemical equations for the preparation of sols : (a) Gold sol by reduction. (b) Fe (OH)3…
A: Write chemical equations for the preparation of sols : (a) Gold sol by reduction. (b) Fe (OH)3
Q: Write the balanced neutralization reaction that occurs between H2SO4 and KOH in aqueous solution.…
A:
Q: 3. During the isolation process sodium carbonate was used and there was an evolution of gas. Write…
A: See the complete solution given below.
Q: Which of the following best describes why magnesium hydroxide, found in milk of magnesia, is soluble…
A: magnesium hydroxide, found in milk of magnesia, is soluble in acidic solution because : According to…
Q: 1. Calculate the molarity of a solution prepared by dissolving 11.5 g of solid NaOH in enogh water…
A: Molarity(M) is the moles of solute per unit volume of the solution in litres. It's unit is mol/L…
Q: The first step in the preparation of magnesium metal is the precipitation of Mg(OH)2 from sea water…
A: At certain temperature, the mixing property of components is determined by their solubility product…
Q: 5. If magnesium hydroxide were used instead of NaOH, how many moles would it take to combine with…
A: We are authorized to answer one question at a time, since you have not mentioned which question you…
Q: 1. What color of precipitate is produced when Na₂O₂ oxidizes Co(OH)₂? 2. What is the product of the…
A: As per our company guidelines we are supposed to answer only three questions.kindly repost remaining…
Q: how do i write the net ionic equation for the formation of Ag2SO4(s)
A: The product of the reaction is shown below, Ag2SO4
Q: Write a balanced chemical equation showing how each of the following metal oxides is dissolved by…
A: The balanced chemical equation can be defined as the reaction having an equal number of atoms…
Q: What is the net ionic equation for the reaction between CoCl2 and an alkali hydroxide?
A: The net ionic equation is a chemical equation in which only species are mentioned which participate…
Q: The Yucca Mountain repository in Nevada, which is intended for the long-term storage of nuclear…
A: Concept introduction- Solubility product (Ksp)- It is equilibrium constant for a chemical reaction…
Q: Why is it possible to use K2S instead of thioacetamide in precipitating the Group II and Group III…
A: Thioacetamide is widely used in classical qualitative inorganic analysis as an in situ source for…
Q: solvent
A: The name of the comlex is Iron(iii) thiocyanate or Ferric Thiocyanate. It dissolves to give Fe3+ and…
Q: Write equation for the reaction of: (i) Iron with steam(ii) Calcium and potassium with water
A:
Q: Can you compare the solubility of Co2 in water compared with saturated NaCl solution?
A: solubility of Co2 in NaCl solution is less than in water increases with the temperature and…
Q: 1. The chemical reaction for biocalcification is: Ca2+ + 2 HCO?=CO2 + CaCO3 + H2O Using this…
A: Biocalcification is related to the accumulation of calcium carbonate to form hard tissue. Oceanic…
Q: Write the balanced equation for this reaction and calculate the molarity of the Al(OH)₃ solution.
A:
Q: Data Report Sheet: The Alkaline Earths and the Halogens A: Oxidizing Power of the Halogens. Color of…
A: A)In the question it is given that - Iodine doesn't give any colour when treated by iodide ion ,…
Q: Each day, the stomach produces 2.0 L of gastric juice that contains 0.10 M HCl. Phillips Milk of…
A: Given that the volume of gastric juice produced is 2L.The molarity of HCl present in gastric juice…
Q: Each day, the stomach produces 2.0 L of gastric juice that contains 0.10 M HCl. Phillips Milk of…
A: Moles of HCl can be calculated as:
Q: write the balanced equation for Lead (II) Nitrate & Ammonium Acetate. What is the white precipitate…
A: The reactants given are Lead (II) Nitrate = Pb(NO3)2 Ammonium Acetate = CH3COONH4
Q: Write the balanced net ionic equation for the precipitation of lead (II) hydroxide from aqueous…
A: A net ionic equation is the chemical equation that shows only those ions, elements and compounds…
Q: Why does the reddish meat become pale when being soak in hydrogen peroxide?
A: The color of the meat is an indicator to decide whether the meat is fresh or not. It is a heme…
Q: Tetraphosphorus decaoxide (P₄O₁₀) is made from phos-phate rock and used as a drying agent in the…
A: The answer for part (a) is given below. Kindly repost the other part as separate question
Q: The equation for the reaction by which a sodium cyanide solution dissolves gold from its ore is…
A: The no of moles of any substance is the ratio of their given mass to the molar mass of that…
Q: Describe in words how you do the following preparation. Then give molecular equation for the…
A: The preparation of MgCl2(s) can be done by reaction of solid Mg(OH)2 with gaseous HCl (hydrochloric…
Q: Calculate the pH of a 0.1 M sodium hypoiodite solution.
A:
Q: 1. Write and balance chemical reactions that happen when Pt is dissolved using aqua regia forming…
A: The chemical reaction for the formation of H2PtCl6 is given below
Q: The salts or hydroxides of transition metals react with diols under basic conditions to form…
A: In coordination chemistry, ligands are molecules that have one or more atoms which can act as Lewis…
Q: The black “smoke” that flows out of deep ocean hydrothermal vents is made of insoluble metal…
A: Given:- The black “smoke” that flows out of deep ocean hydrothermal vents is made of insoluble metal…
Q: The color of the precipitate formed when phosphate ion reacts with reagent added in the test for…
A: The test for presence of phosphate ion is done by the use of reagent ammonium heptamolybdate.
Q: Write the balanced net ionic equation for the precipitation of magnesium sulfide from aqueous…
A: Welcome to bartleby ! We have to write balanced net ionic equation for formation of Magnessium…
Q: Ammonia is added when preparing calcium ?oxalate. Why
A: Interpretation - To tell about why ammonia is added to preparing calcium oxalate -…
Q: 3. Which ion is formed during nitration with a mixture of nitric and sulfuric acid? a. HSO, b. NO2…
A: Given, Which ion is formed during nitration with a mixture of nitric and sulfuric acid ? Options…
Q: The group III ions are separated from each other by taking advantage of the fact that two of the…
A: An amphoteric hydroxide is defined as a hydroxide that behaves as both a weak acid as well as a weak…
Q: Write the reaction equation for the test for carbon dioxide with calcium hydroxide solution. Show…
A: When Carbon dioxide (CO2 (g)) reacts with Calcium hydroxide solution ((Ca(OH)2 (aq), Lime water)…
Q: Give’ TWO large scale uses each, of sulphuric acid and nitric acid
A: Sulphuric acid has the formula H2SO4. It is commonly used chemical in industry which is prepared by…
Q: Can the metal activity series explain why potassium metal can be prepared from the reaction of…
A: The metal activity series also called as the activity series is defined as the arrangement of metals…
Q: Write chemical equations for the preparation of sols :(a) Gold sol by reduction.(b) hydrated ferric…
A: Given preparations, (a) Gold sol by reduction.(b) hydrated ferric oxide sol by hydrolysis
Q: During bad storage of cement the alkalis in the cement react with CO2 to form alkali carbonates,…
A: A cement is a substance that is used for construction that sets, hardens, and adheres to other…
Q: A sample of an industrial waste water is analyzed and found to contain 27.0 ppb Cr³+. How many grams…
A: Given that - ppb Concentration of Cr3+ in industrial waste water = 27.0 ppb Total mass of waste…
Q: You can fit 25 drops of substance X on a penny and 12 drops of substance Y on a different penny.…
A: More the number of Penny's means they are stick to each other. Hence, stronger the intermolecular…
1. Write a balanced
2. Why is AgNO3 used to test the presence of Cl? Write the chemical reaction.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Balance these equations by providing the missingcoefficients:(a) __ Fe1s2 + __O21g2¡__Fe2O31s2(b) __ Al1s2 + __ HCl1aq2¡__ AlCl31aq2 + __ H21g2(c) __ CaCO31s2 + __ HCl1aq2¡__ CaCl21aq2 +__ CO21g2 + __ H2O1l2Calculate for the theoretical yield of Rochelle salt (MW = 282.83) (a) that will be obtained from 6.6 grams of monohydrated Sodium carbonate (124.01). What is the reacting ratio? Refer to the choices below. (b) that will be obtained from 15g of Potassium bitartrate (MW = 188.10). Refer to the choices below. Rochelle salt: 2KHC4H4O6 + Na2CO3.H2O + 6H2O ---->2 C4H4O6KNa.4H2O +CO2 A 22.55 g B 25.47 g C 30.11 g D 32.64 gA weight of 0.50 g was taken impure container containing sodium carbonate and bicarbonate. Dissolved in water and then crushed with hydrochloric acid (0.1 N), the burette reading game was at the endpoint of phenolphthalein of 10.5 ml and at the end point of the orange methylation point 30.1 ml. The percentage of sodium carbonate was in ................. knowing that the weights are: Na: 23, C: 12, O: 16
- In the synthesis of benzoic acid, 3.5 mL of toluene were used and mixed with potassium permanganate solution. In making the potassium permanganate solution, 7 grams of the powder were dissolved in 150 mL of water. The resulting crystals were purified and the yield 1.53 grams. Identify the limiting reagent and compute for the number of moles that it consumed. What is the theoretical yield? What is the percentage yield? MW toluene = 94.14, density=0.87 g/mL , MW KMnO4 = 158, density = 2.7 g/mL , MW Benzoic acid = 122, density = 1.27 g/mLPls give with reason, the type and name of the reaction.Solid residue weighing 7.158 g from an aluminum refining process was dissolved in acid to give Al3+ in solution. The solution was treated with 8-hydroxyquinoline to precipitate (8-hydroxyquinoline)3Al (FM 462.462 g/mol), which was ignited to give Al2O3 (FM 101.961 g/mol) weighing 1.178 g. Find the weight percent of Al (FM 26.982 g/mol) in the original mixture.
- Potassium dichromate has several industrial applications. To determine the purity of the salt that will be used in different industrial processes, a sample mass equal to 2.660 g was dissolved and quantitatively transferred to a 500.00 mL flask. An aliquot of 25.00 mL of this solution was treated with excess KI and the released iodine was titrated with 0.1000 mol L-1 sodium thiosulfate, spending 27.00 mL. Calculate the purity of the analyzed salt. Data:K = 39.10 O = 16.00 Cr = 52.00 I = 126.9 S = 32.077 determination of sodium, potassium and calcium in water samples by ICP-OES. 1. kindly assist me with discussion in the experiment 2. results of the experiment 3 experimental/ methodologyCalcium oxalate monohydrate [Ca(O2CCO2)·H2O, also written as CaC2O4·H2O] is a sparingly soluble salt that is the other major component of kidney stones [along with Ca3(PO4)2]. Its solubility in water at 25°C is 7.36 × 10−4 g/100 mL. Calculate its Ksp. MW CaC2O4·H2O = 146.1 g/mol
- Congratulations, you have just been hired at a local laboratory. Your first task is to prepare 10.0 mL of a 1.50 x 10-6 M solution of a small protein (FW: 3600 g/mole). Available to you are an electronic balance that is accurate to the nearest 0.001 g, a 1.00-mL pipette, and 10-mL volumetric flasks. Describe how to prepare the desired solution. (Note that the amount of protein is so small that you will need to make a more concentrated stock solution and dilute it.)Uranium as U(VI) (as the uranyl ion; UO22+) is soluble and moves easily with groundwater, but U(VI) (as uraninite; UO2) is relatively insoluble. Several microorganisms are able to mediate this process. As such, in many uranium-contaminated aquifers, the reduction of soluble uranyl to insoluble uraninite by microorganisms has been proposed as a way to remove the dissolved uranium from water and keep it “locked up” in the subsurface. If the U(VI) is primarily complexed with carbonate (as UO2(CO3)34-), how does that influence the energy yield (or “reducibility”) of the U(VI), assuming “standard” conditions (i.e. not taking concentrations of any products or reactants into account? What about at pH 7? Under what kind of conditions would you expect to find UO2(CO3)34- vs UO22+ (and why)?Yttrium (III) carbonate (MM = 357.84 g/mol) has a Ksp of 1.0 x10-31. If 15.5 g Y2(CO3)3 is stirred into 2.16 L H2O, how many micrograms of yttrium (III) carbonate will dissolve?Report your answer to the nearest whole number.

