1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 78 1.9 2.0 222 2.1 22 0.04 0.06 0.09 0.11 0.15 0.19 0.24 0.30 0.38 0.48 0.60 0.75 0.92 1.14 1.41 1.72 2.09 2.52 3.02 3.58 4.19 4.86 5.56 6.27 6.99 7.69 8.35 8.96 9.51 10.00 10.43 10.80 11.11 11.37 11.50 11.76 11.91 12.02 12 12 12.20 12 26 12.31 12:35 12.38 12.40 12:42 1244 10.2 10.1 10.0 9.9 98 9.7 9.6 9.5 94 9.3 92 9.1 9.0 8.9 8.7 86 85 8.4 83 82 8.1 80 7.9 7.8 7.7 76 75 7.4 7.3 72 71 70 69 6.8 6.7 66 6.5 64 6.3 62 61 60 5.9 5.8 5.7 56 12.48 12.49 12.49 12.50 12.50 12.50 12.51 12.51 12.52 12.52 12.53 12.54 12.55 12.56 12.57 12.59 12.62 12.65 12.69 12.74 12.80 12.88 12.98 13.11 13.28 13.49 13.77 14.12 14.59 15.21 16.04 17.19 18.81 21.20 5.1 5.0 4.9 4.8 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 3222 28 2.7 2.6 54321098 2.5 2222 24 2.3 2.2 2.1 20 1.9 1.8 0.23 0.32 042 055 0.70 0.89 1.12 1.40 1.74 2.16 266 3.26 3.97 481 5.77 6.85 8.05 9.36 10.74 12 17 1361 15.01 16.36 17.61 18.75 19.77 2066 21.42 22.07 2262 23.07 2344 23.75 23.99 24.19 24.36 24.49 24.50 24.67 24.74 24.79 24.84 24.87 24.90 24.92 24.93 24.95 24.96 24.97 24.98 24.96 24.99 36 37 38 39 40 42 43 44 45 46 47 48 50 5.1 52 53 54 5.5 56 57 5.8 5.9 60 6.1 62 63 64 65 66 67 68 69 7.0 71 72 73 74 7.5 76 7.7 78 79 80 8.1 82 83 84 85 25.00 25.00 25.01 25.01 25.01 25.02 25.02 25.03 25.04 25.05 25.06 25.08 25.10 25.13 25.16 25.20 25.25 25.32 25.40 25.50 25.64 25.81 26.02 26.29 26.63 27.07 27.64 28.37 29.31 30.56 32.20 34.42 37.46 41.77 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 9.9 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 10.9 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 12.0 12.1 12.2 12.3 12.4
1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 78 1.9 2.0 222 2.1 22 0.04 0.06 0.09 0.11 0.15 0.19 0.24 0.30 0.38 0.48 0.60 0.75 0.92 1.14 1.41 1.72 2.09 2.52 3.02 3.58 4.19 4.86 5.56 6.27 6.99 7.69 8.35 8.96 9.51 10.00 10.43 10.80 11.11 11.37 11.50 11.76 11.91 12.02 12 12 12.20 12 26 12.31 12:35 12.38 12.40 12:42 1244 10.2 10.1 10.0 9.9 98 9.7 9.6 9.5 94 9.3 92 9.1 9.0 8.9 8.7 86 85 8.4 83 82 8.1 80 7.9 7.8 7.7 76 75 7.4 7.3 72 71 70 69 6.8 6.7 66 6.5 64 6.3 62 61 60 5.9 5.8 5.7 56 12.48 12.49 12.49 12.50 12.50 12.50 12.51 12.51 12.52 12.52 12.53 12.54 12.55 12.56 12.57 12.59 12.62 12.65 12.69 12.74 12.80 12.88 12.98 13.11 13.28 13.49 13.77 14.12 14.59 15.21 16.04 17.19 18.81 21.20 5.1 5.0 4.9 4.8 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 3222 28 2.7 2.6 54321098 2.5 2222 24 2.3 2.2 2.1 20 1.9 1.8 0.23 0.32 042 055 0.70 0.89 1.12 1.40 1.74 2.16 266 3.26 3.97 481 5.77 6.85 8.05 9.36 10.74 12 17 1361 15.01 16.36 17.61 18.75 19.77 2066 21.42 22.07 2262 23.07 2344 23.75 23.99 24.19 24.36 24.49 24.50 24.67 24.74 24.79 24.84 24.87 24.90 24.92 24.93 24.95 24.96 24.97 24.98 24.96 24.99 36 37 38 39 40 42 43 44 45 46 47 48 50 5.1 52 53 54 5.5 56 57 5.8 5.9 60 6.1 62 63 64 65 66 67 68 69 7.0 71 72 73 74 7.5 76 7.7 78 79 80 8.1 82 83 84 85 25.00 25.00 25.01 25.01 25.01 25.02 25.02 25.03 25.04 25.05 25.06 25.08 25.10 25.13 25.16 25.20 25.25 25.32 25.40 25.50 25.64 25.81 26.02 26.29 26.63 27.07 27.64 28.37 29.31 30.56 32.20 34.42 37.46 41.77 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 9.9 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 10.9 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 12.0 12.1 12.2 12.3 12.4
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter1: Chemistry: An Introduction
Section: Chapter Questions
Problem 6ALQ: Scientific models do not describe reality. They are simplifications aid therefore incorrect at some...
Related questions
Question
Determine the pKa values for your weak acid and weak base equivalence points using the pH obtained at the half equivalence point for each graph. Label the pKa’s (and volume) on your graphs.
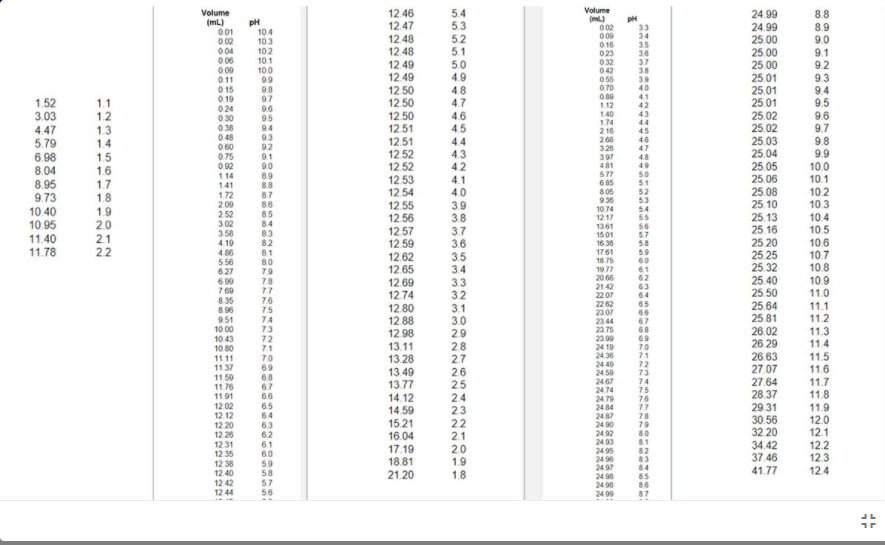
Transcribed Image Text:Volume
(mL)
001
0 02
004
0 06
0 09
011
12.46
12.47
12.48
12.48
12 49
12.49
Volume
(m)
002
009
0 16
023
032
042
055
070
5.4
5.3
24.99
24.99
25.00
25.00
25.00
25.01
25.01
25.01
25.02
25.02
25.03
25.04
25.05
25.06
8.8
pH
10.4
10.3
102
10.1
100
90
98
97
96
95
8.9
9.0
9.1
33
5.2
5.1
50
4.9
4.8
4.7
4.6
4.5
4.4
4.3
4.2
9.2
38
39
40
9.3
9.4
9.5
0 15
0 19
024
030
038
12.50
12.50
41
1.52
1.1
1.12
1.40
1.74
216
266
326
397
481
577
685
8 05
936
10.74
12 17
1361
1501
16.36
17.61
18.75
19 77
20 66
21 42
2207
22 62
2307
23 44
2375
23 99
24 19
24 36
24 49
24 50
24 67
24.74
24 79
24 84
2487
24 90
24 92
24 93
24 95
24 96
24 97
24 98
24 98
24 99
42
43
3.03
4.47
1.2
12.50
12.51
12.51
12.52
12.52
12.53
12.54
12.55
12.56
44
9.7
1.3
1.4
94
9.3
92
9.1
9.0
8.9
88
8.7
86
8.5
84
83
82
81
80
7.9
78
7.7
76
75
7.4
7.3
72
7.1
70
69
68
6.7
66
6.5
64
63
62
6.1
6.0
59
45
46
47
48
49
50
51
52
53
54
55
56
0 48
0 60
0.75
0.92
1.14
141
1.72
209
2.52
3 02
3.58
4.19
4.86
5.56
627
6 99
7 69
8.35
8.96
9.51
10 00
10 43
10 80
11.11
11.37
11.50
11.76
11 91
12.02
12 12
12 20
12 26
12 31
12 35
9.8
9.9
10.0
5.79
6.98
8.04
1.5
1.6
8.95
9.73
1.7
1.8
4.1
4.0
3.9
25.08
25.10
10.1
10.2
10.3
10.40
1.9
25.13
25.16
25.20
25.25
25.32
25.40
10.4
10.5
10.6
10.7
10.8
10.9
11.0
3.8
10.95
11.40
11.78
2.0
2.1
12.57
12.59
3.7
3.6
58
59
60
61
62
63
64
65
66
67
68
69
7.0
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
22
12.62
12.65
12 69
12.74
12.80
12.88
12.98
13.11
3.5
3.4
3.3
3.2
3.1
3.0
2.9
2.8
25.50
25.64
11.1
11.2
11.3
11.4
25.81
26.02
26.29
26.63
11.5
11.6
11.7
11.8
11.9
12.0
12.1
13.28
2.7
13.49
27.07
2.6
2.5
2.4
27.64
28.37
29.31
30.56
32.20
34.42
37.46
41.77
13.77
14.12
14.59
1521
22
16.04
2.1
12 38
12 40
12 42
12 44
17.19
18.81
21.20
2.0
1.9
122
12.3
12.4
58
5.7
56
1.8
9899
O O76 5432N 10900
5555
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning