Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter20: Chemistry Of Selected Transition Elements And Coordination Compounds
Section: Chapter Questions
Problem 105QRT
Related questions
Question
100%
Please answer very soon will give rating surely
Complete Answer needed
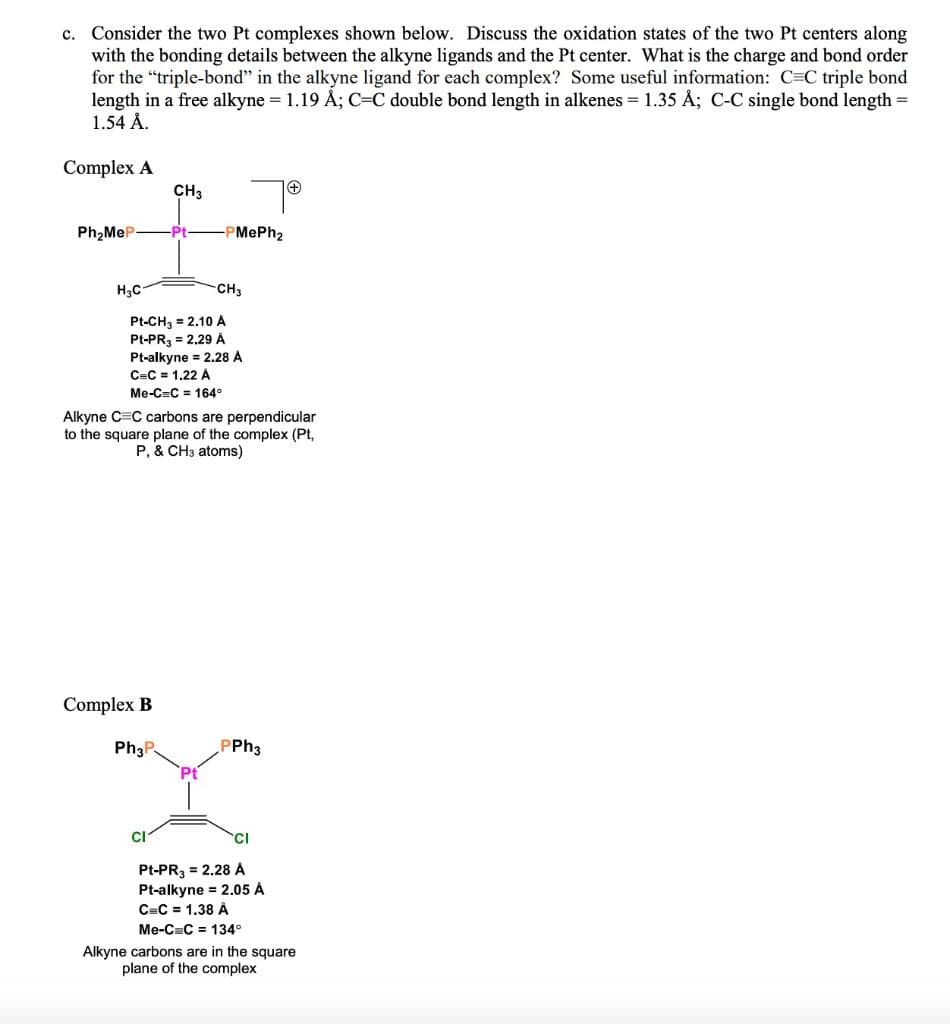
Transcribed Image Text:c. Consider the two Pt complexes shown below. Discuss the oxidation states of the two Pt centers along
with the bonding details between the alkyne ligands and the Pt center. What is the charge and bond order
for the "triple-bond" in the alkyne ligand for each complex? Some useful information: C=C triple bond
length in a free alkyne = 1.19 Å; C=C double bond length in alkenes = 1.35 Å; C-C single bond length =
1.54 Å.
Complex A
CH3
Ph2MeP-
Pt
-PMePh2
H3C
CH3
Pt-CH, = 2.10 A
Pt-PR, = 2.29 Á
Pt-alkyne = 2.28 A
C=C = 1,22 A
Me-C=C = 164°
Alkyne C=C carbons are perpendicular
to the square plane of the complex (Pt,
P, & CH3 atoms)
Complex B
Ph3P
PPH3
CI
Pt-PR3 = 2.28 A
Pt-alkyne = 2.05 Á
C=C = 1.38 Â
Me-C=C = 134°
Alkyne carbons are in the square
plane of the complex
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
