1.Consider the polymerization of the following monomers: H2N- -NH2 CI (a) Draw the repeat unit of the resulting polymer. (b) Identify the two structural units comprising the repeat unit.
1.Consider the polymerization of the following monomers: H2N- -NH2 CI (a) Draw the repeat unit of the resulting polymer. (b) Identify the two structural units comprising the repeat unit.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 51QAP
Related questions
Question
100%
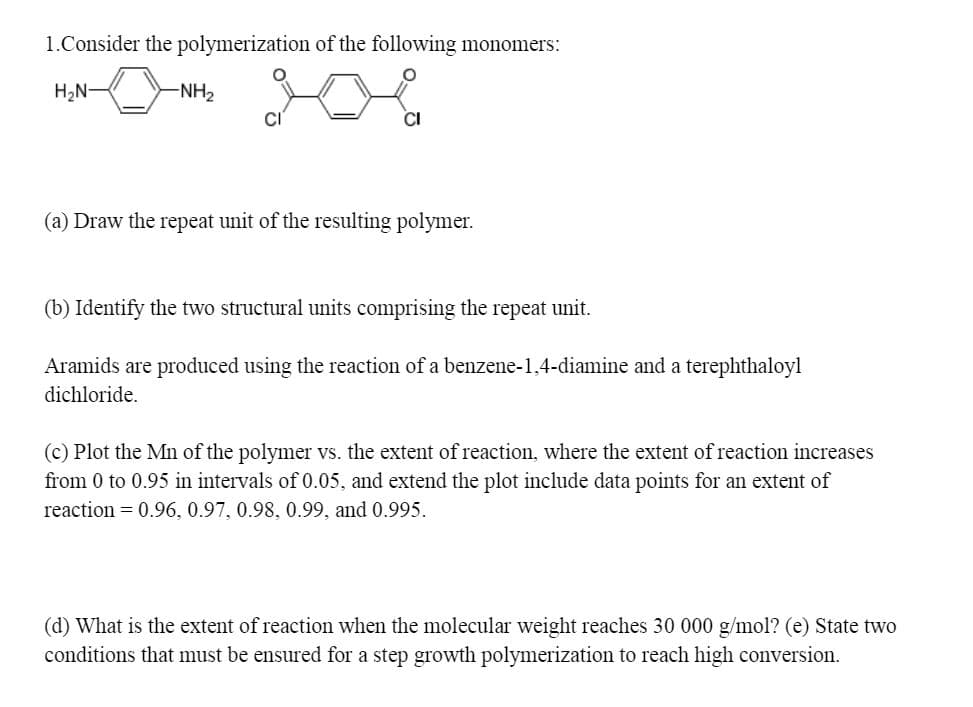
Transcribed Image Text:1.Consider the polymerization of the following monomers:
H2N-
-NH2
(a) Draw the repeat unit of the resulting polymer.
(b) Identify the two structural units comprising the repeat unit.
Aramids are produced using the reaction of a benzene-1,4-diamine and a terephthaloyl
dichloride.
(c) Plot the Mn of the polymer vs. the extent of reaction, where the extent of reaction increases
from 0 to 0.95 in intervals of 0.05, and extend the plot include data points for an extent of
reaction = 0.96, 0.97, 0.98, 0.99, and 0.995.
(d) What is the extent of reaction when the molecular weight reaches 30 000 g/mol? (e) State two
conditions that must be ensured for a step growth polymerization to reach high conversion.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning