10-8. < PREV NEXT > 2 3 Based on the result of the acid-base reaction, set up the ICE table in order to determine the unknown. HCIO(aq) H,O(1) H,Oʻ(aq) CIO-(aq) Initial (M) Change (M) Equilibrium (M) RESET
10-8. < PREV NEXT > 2 3 Based on the result of the acid-base reaction, set up the ICE table in order to determine the unknown. HCIO(aq) H,O(1) H,Oʻ(aq) CIO-(aq) Initial (M) Change (M) Equilibrium (M) RESET
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
I have no clue how to fill out this ICE table at all.
Transcribed Image Text:Bb Chem 101 - Blackboard Learn
A learn.cnm.edu/webapps/blackboard/content/contentWrapper.jsp?content_id=_9803514_1&displayName=Chem+101&course_id=_161163_1&navltem=content&href=%2Fwebapps%2Fblackboard%2F. *
CNM Folder
Chemistry Folder
School Folder
Media Folder
Extras
(1) Dashboard | Kha..
2 ALIVIA ABERNATHY
48
CNM>Learn
CNM
Courses
Community
Question 9 of 36
Submit
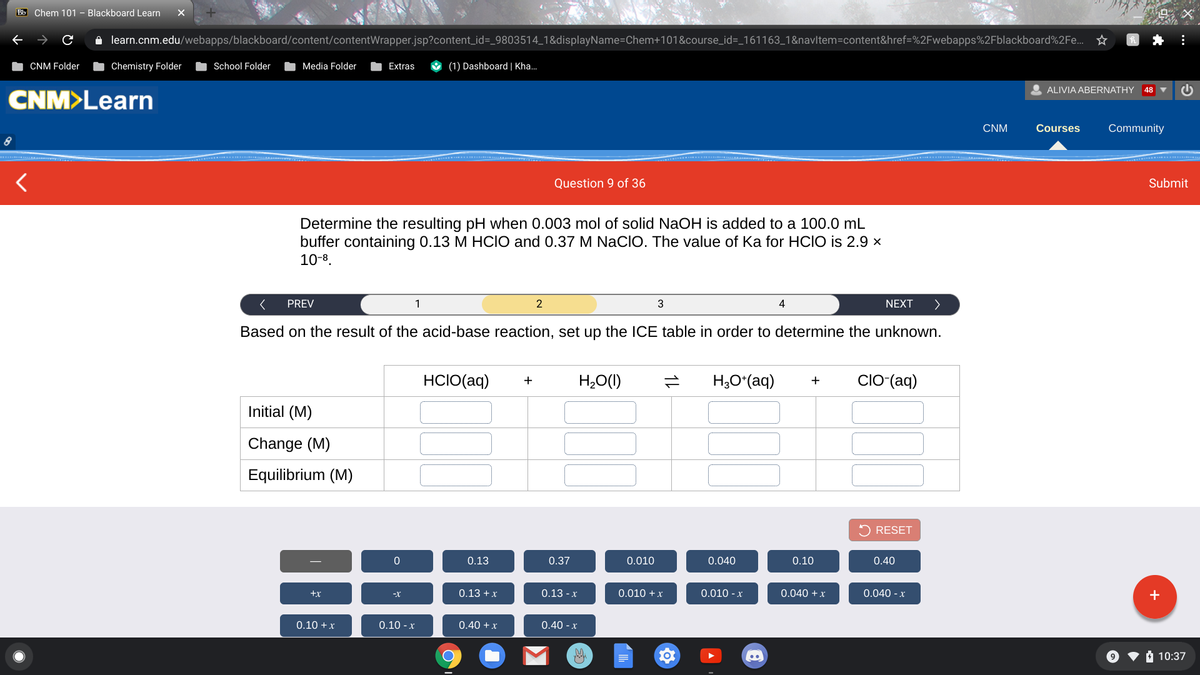
Determine the resulting pH when 0.003 mol of solid NaOH is added to a 100.0 mL
buffer containing 0.13 M HCIO and 0.37 M NaCIO. The value of Ka for HCIO is 2.9 x
10-8.
PREV
1
2
3
NEXT
>
Based on the result of the acid-base reaction, set up the ICE table in order to determine the unknown.
HCIO(aq)
H,O()
H;O*(aq)
ClO-(aq)
+
+
Initial (M)
Change (M)
Equilibrium (M)
5 RESET
0.13
0.37
0.010
0.040
0.10
0.40
0.13 +x
0.13 -x
0.010 +x
0.010 - x
0.040 + x
0.040 - x
+
+x
-X
0.10 +x
0.10 - x
0.40 +x
0.40 - x
1 10:37
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



