10) A sample of nitrogen gas is placed in a sealed piston that has a volume of 1.8 L. The sample exerts a pressure of 1.4 atm at 25 °C. What is the pressure in the piston if the temperature is kept constant, but the volume is reduced to 0.9 L? A) 0.7 atm B) 0.9 atm C) 1.4 atm D) 1.8 atm E) 2.8 atm
10) A sample of nitrogen gas is placed in a sealed piston that has a volume of 1.8 L. The sample exerts a pressure of 1.4 atm at 25 °C. What is the pressure in the piston if the temperature is kept constant, but the volume is reduced to 0.9 L? A) 0.7 atm B) 0.9 atm C) 1.4 atm D) 1.8 atm E) 2.8 atm
Chapter11: The Air Around Us
Section: Chapter Questions
Problem 44E
Related questions
Question
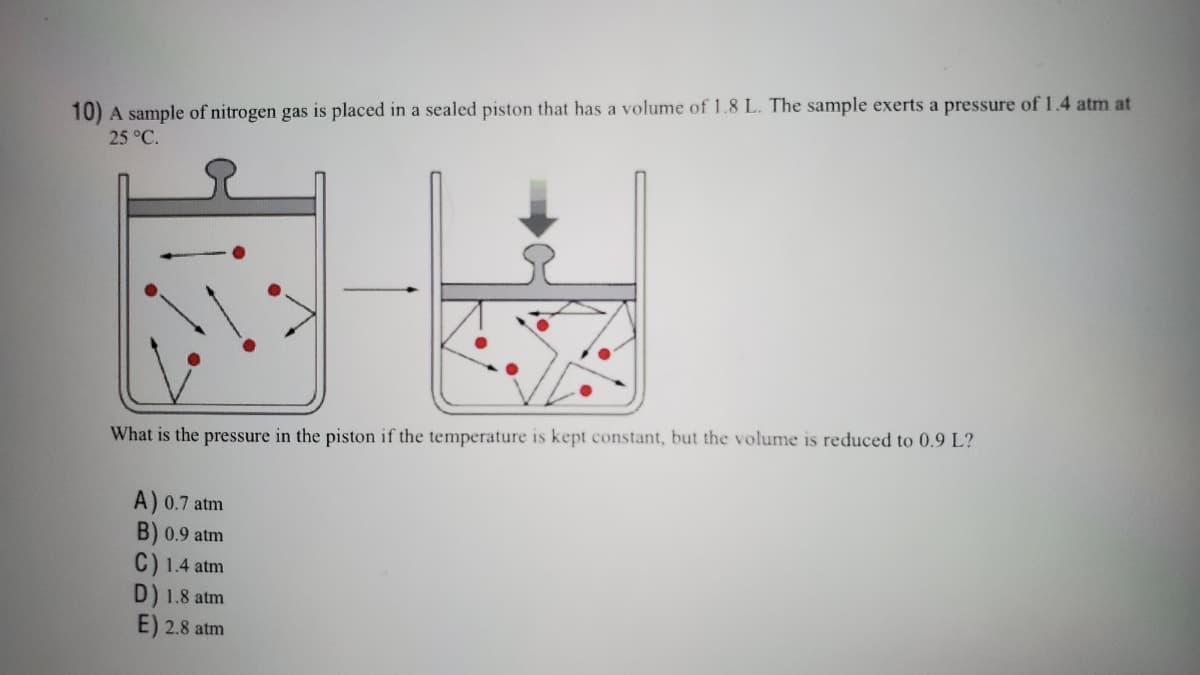
Transcribed Image Text:10) A sample of nitrogen gas is placed in a sealed piston that has a volume of 1.8 L. The sample exerts a pressure of 1.4 atm at
25 °C.
What is the pressure in the piston if the temperature is kept constant, but the volume is reduced to 0.9 L?
A) 0.7 atm
B) 0.9 atm
C) 1.4 atm
D) 1.8 atm
E) 2.8 atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
