Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section11.6: Properties Of Liquids
Problem 1.2ACP
Related questions
Question
10) Calculate the moles of magnesium ( Show your calculation)
Transcribed Image Text:4/Downloads/CHM%20111%20Empirical%20Formula%20.pdf
++
14
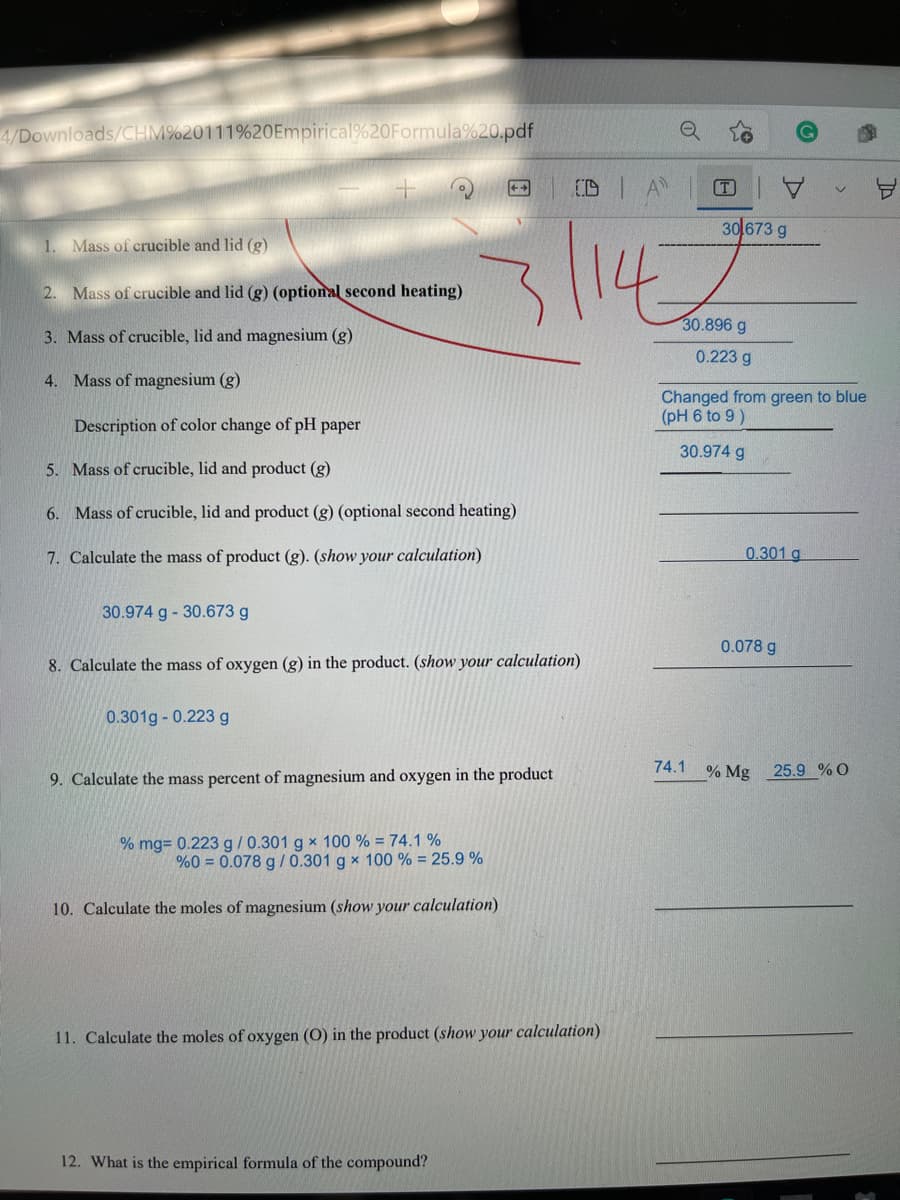
30 673 g
1. Mass of crucible and lid (g)
2. Mass of crucible and lid (g) (optional second heating)
30.896 g
3. Mass of crucible, lid and magnesium (g)
0.223 g
4. Mass of magnesium (g)
Changed from green to blue
(pH 6 to 9)
Description of color change of pH paper
30.974 g
5. Mass of crucible, lid and product (g)
6. Mass of crucible, lid and product (g) (optional second heating)
7. Calculate the mass of product (g). (show your calculation)
0.301 g
30.974 g - 30.673 g
0.078 g
8. Calculate the mass of oxygen (g) in the product. (show your calculation)
0.301g - 0.223 g
74.1 % Mg
25.9 % O
9. Calculate the mass percent of magnesium and oxygen in the product
% mg= 0.223 g/0.301 g x 100 % = 74.1 %
%0 = 0.078 g /0.301 g x 100 % = 25.9 %
10. Calculate the moles of magnesium (show your calculation)
11. Calculate the moles of oxygen (O) in the product (show your calculation)
12. What is the empirical formula of the compound?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning