i. The ionization energy of fluorine (F) is ~1700 kJ/mol, while the ionization energy of difluorine (F2) is ~1500 kJ/mol. ii. Bez cannot exist, while both Be2* and Be,¯ can exist. The longest wavelength of light that can be absorbed by N2 is ~80 nm, while the longest wavelength of light that can be absorbed by F2 is ~100 nm. (No calculations are required for this). ii.
i. The ionization energy of fluorine (F) is ~1700 kJ/mol, while the ionization energy of difluorine (F2) is ~1500 kJ/mol. ii. Bez cannot exist, while both Be2* and Be,¯ can exist. The longest wavelength of light that can be absorbed by N2 is ~80 nm, while the longest wavelength of light that can be absorbed by F2 is ~100 nm. (No calculations are required for this). ii.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 119CP
Related questions
Question
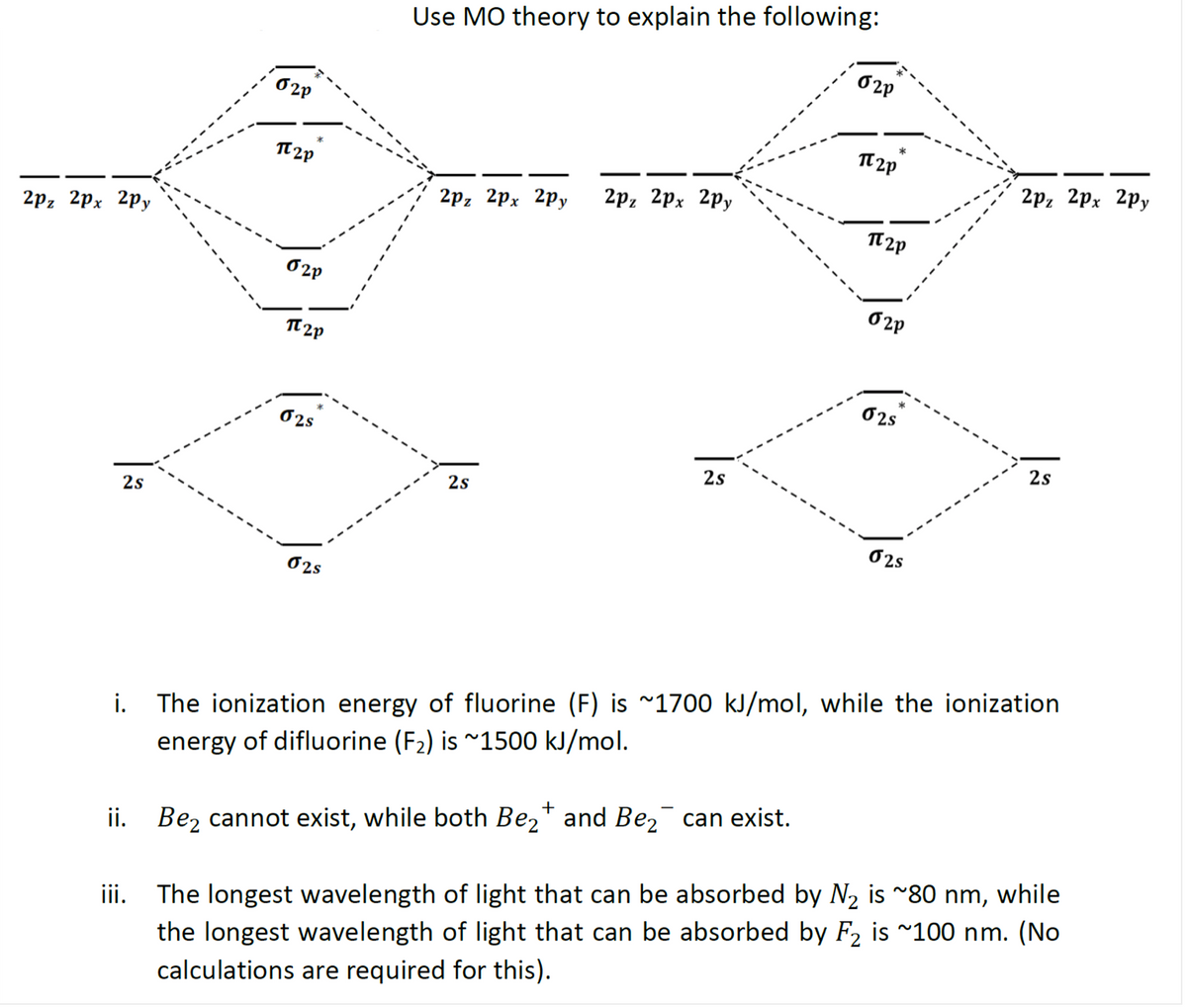
Transcribed Image Text:Use MO theory to explain the following:
O2P
*
2p, 2pх 2ру
TT 2p
2р, 2рx 2ру
2р, 2рх 2ру
2р, 2рх 2ру
O 2P
O 2P
TT 2p
O2s
02s
2s
2s
2s
2s
O2s
O2s
i. The ionization energy of fluorine (F) is ~1700 kJ/mol, while the ionization
energy of difluorine (F2) is ~1500 kJ/mol.
+
iii. The longest wavelength of light that can be absorbed by N2 is ~80 nm, while
the longest wavelength of light that can be absorbed by F2 is ~100 nm. (No
calculations are required for this).
ii. Bez cannot exist, while both Be,* and Be,¯ can exist.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning