Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 98QRT: Does the pH of the solution increase, decrease, or stay the same when you (a) Add solid sodium...
Related questions
Question
10) help
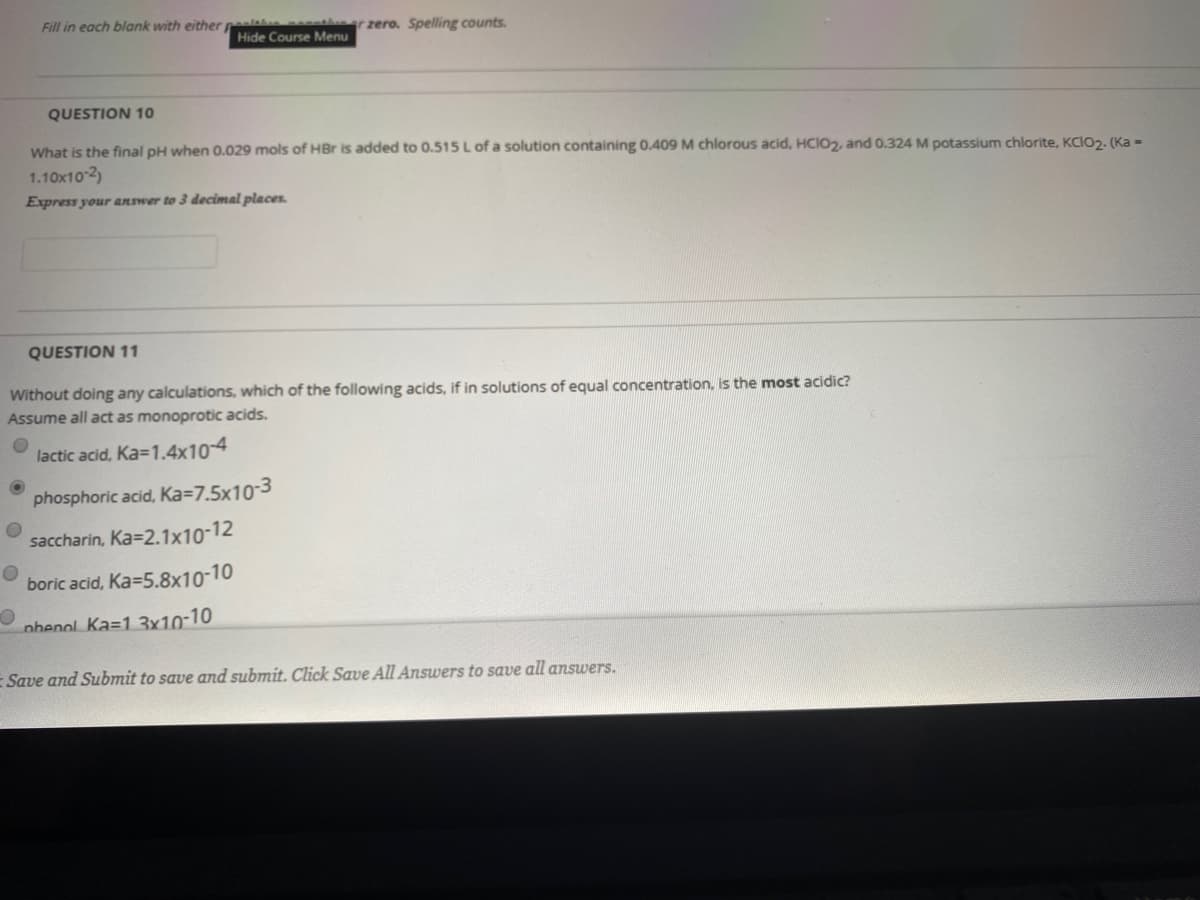
Transcribed Image Text:Fill in each blank with either peala et
Hide Course Menu
rzero. Spelling counts.
QUESTION 10
What is the final pH when 0.029 mols of HBr is added to 0.515 L of a solution containing 0.409 M chlorous acid, HCIO2, and 0.324 M potassium chlorite, KCIO2. (Ka =
1.10x10 2)
Express your answer to 3 decimal places.
QUESTION 11
Without doing any calculations, which of the following acids, if in solutions of equal concentration, is the most acidic?
Assume all act as monoprotic acids.
lactic acid, Ka=1.4x10-4
phosphoric acid, Ka=7.5x10-3
saccharin, Ka=2.1x10-12
boric acid, Ka=5.8x10-10
nhenol. Ka=1 3x10-10
E Save and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning