10. A physician orders magnesium sulfate to be administered at a rate of 2 g/h to a patient experiencing premature labor. The pharmacy sends 500 mL of a 2% solution. What is the osmolarity of the solution? How many mEq per hour of magnesium will this patient receive? MW (MgSO,): 120 g/mol
10. A physician orders magnesium sulfate to be administered at a rate of 2 g/h to a patient experiencing premature labor. The pharmacy sends 500 mL of a 2% solution. What is the osmolarity of the solution? How many mEq per hour of magnesium will this patient receive? MW (MgSO,): 120 g/mol
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question
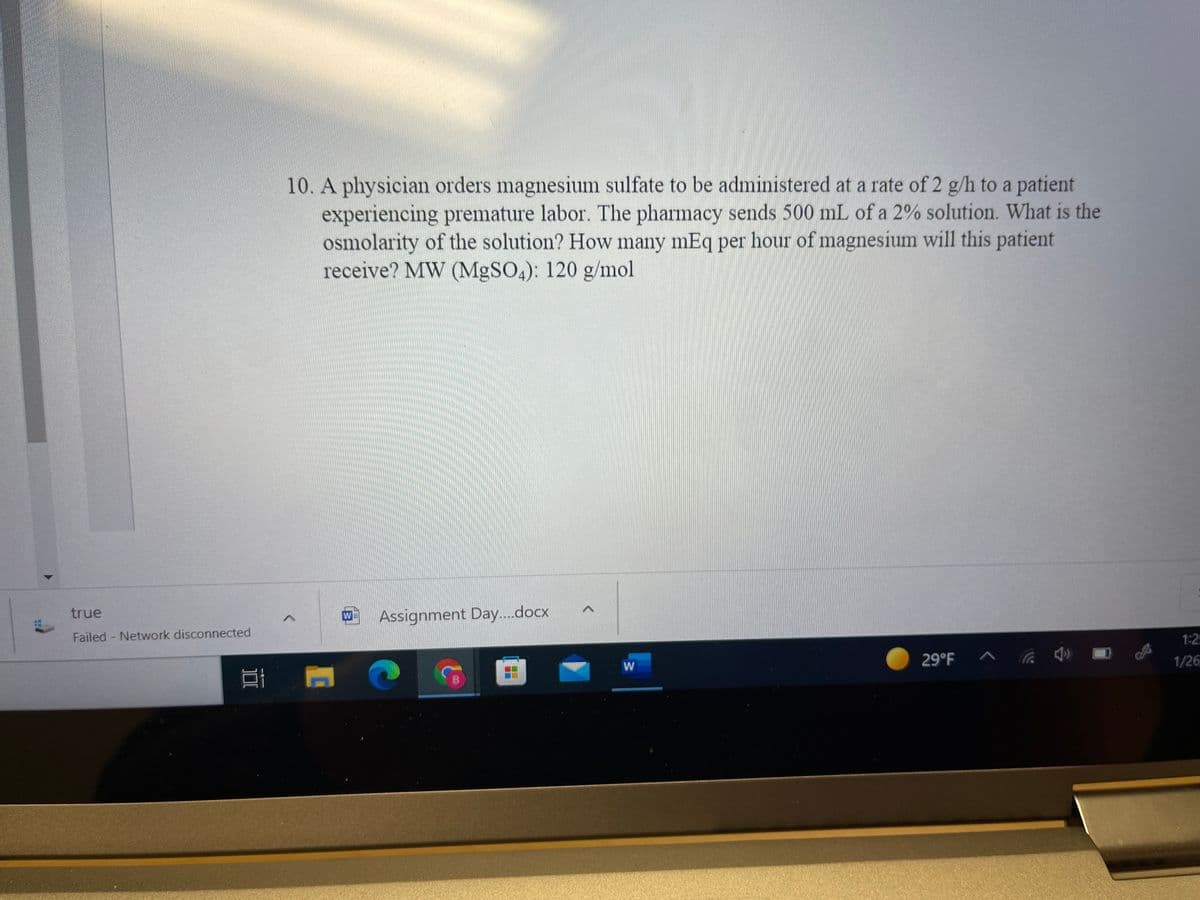
Transcribed Image Text:10. A physician orders magnesium sulfate to be administered at a rate of 2 g/h to a patient
experiencing premature labor. The pharmacy sends 500 mL of a 2% solution. What is the
osmolarity of the solution? How many mEq per hour of magnesium will this patient
receive? MW (MgSO4): 120 g/mol
true
Assignment Day..docx
W
Failed - Network disconnected
1:2
29°F ^
1/26
W
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning