10. Classify the following reactions according to their reaction type. а. 4 Cr 3 O2 2 CrrO3 b. FeCl3 + 3 AGNO; → 3 AgCl + Fe(NO;); c. 4 H;PO4 → P,O10 + 6 H2O d. Zn + 2 HCI → H2 + ZnCl2
10. Classify the following reactions according to their reaction type. а. 4 Cr 3 O2 2 CrrO3 b. FeCl3 + 3 AGNO; → 3 AgCl + Fe(NO;); c. 4 H;PO4 → P,O10 + 6 H2O d. Zn + 2 HCI → H2 + ZnCl2
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter6: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 42E: Suppose 50.0 mL of 0.250 M CoCl2 solution is added to 25.0 mL of 0.350 M NiCl2 solution. Calculate...
Related questions
Question
100%
Classify the following reactions according to their reaction type.
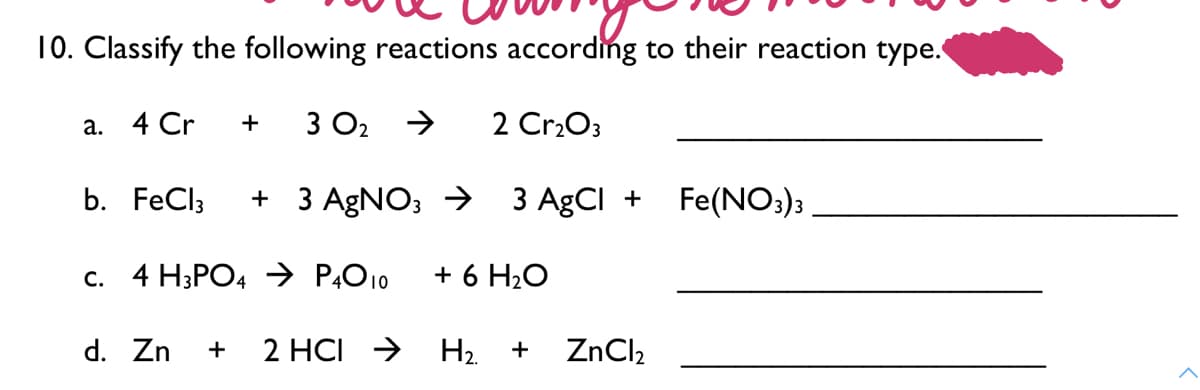
Transcribed Image Text:10. Classify the following reactions according to their reaction type.
а. 4 Cr
+
3 O2
2 Cr2O3
b. FeCl3
+ 3 AGNO; → 3 AgCI +
Fe(NO:)3
c. 4 H;PO4 → P,O10
+ 6 H2O
d. Zn
2 HCI >
H2.
ZnCl,
+
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning