10.57 In addition to ammonia, nitrogen forms three other hydrides: hydrazine (N,H), diazene (N,H,), and tetrazene (N,H,). (a) Use Lewis structures to compare the strength, length, and order of the nitrogen-nitrogen bonds in hydrazine, diazene, and N2. (b) Tetrazene (atom sequence H,NNNNH,) decomposes above 0°C to hydrazine and nitrogen gas. Draw a Lewis structure for tetrazene, and calculate AHn for this decomposition. rxn
10.57 In addition to ammonia, nitrogen forms three other hydrides: hydrazine (N,H), diazene (N,H,), and tetrazene (N,H,). (a) Use Lewis structures to compare the strength, length, and order of the nitrogen-nitrogen bonds in hydrazine, diazene, and N2. (b) Tetrazene (atom sequence H,NNNNH,) decomposes above 0°C to hydrazine and nitrogen gas. Draw a Lewis structure for tetrazene, and calculate AHn for this decomposition. rxn
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 38P: The percent ionic character of the bonds in several interhalogen Molecules (as estimated from their...
Related questions
Question
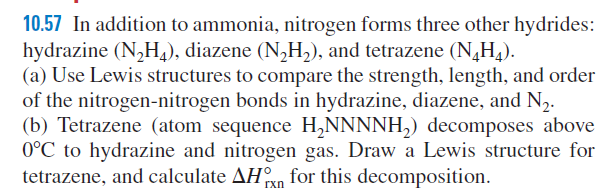
Transcribed Image Text:10.57 In addition to ammonia, nitrogen forms three other hydrides:
hydrazine (N,H), diazene (N,H,), and tetrazene (N,H,).
(a) Use Lewis structures to compare the strength, length, and order
of the nitrogen-nitrogen bonds in hydrazine, diazene, and N2.
(b) Tetrazene (atom sequence H,NNNNH,) decomposes above
0°C to hydrazine and nitrogen gas. Draw a Lewis structure for
tetrazene, and calculate AHn for this decomposition.
rxn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning