Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter12: Atomic X-ray Spectrometry
Section: Chapter Questions
Problem 12.8QAP
Related questions
Question
11
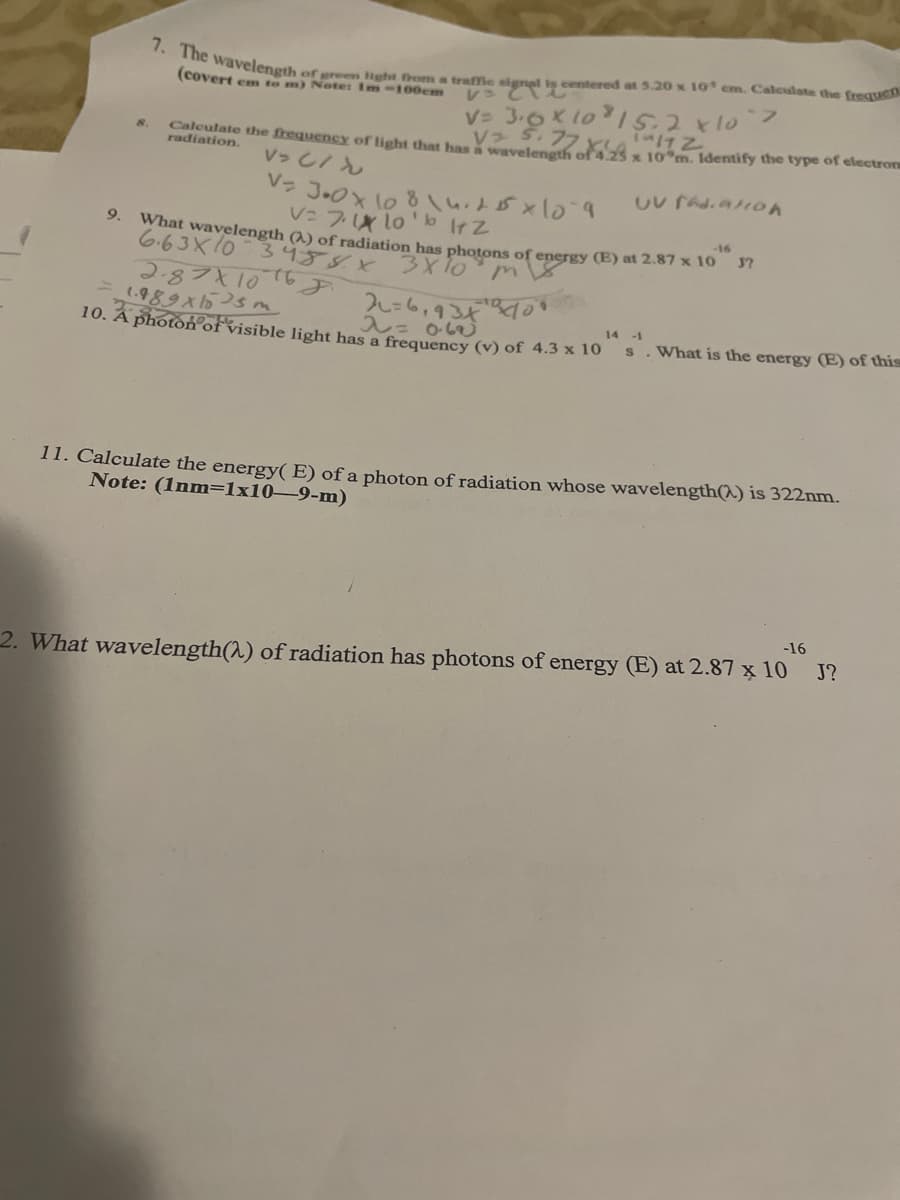
Transcribed Image Text:7. The wavelength
of green Hght from a traffie signal is centered at 5.20 x 1I0 cm. Caleulate the frequen
レ でむ
V= 3.0K 1o15.2x10
V S.77
(covert em to m) Note: Im-100cm
フ
Caleulate the frequency of light that has waveleneth of 4.25 x 10 m. Identify the type of electroru
radiation.
8.
V>し/ん
V: 7 lo'b IrZ
9.
-16
What wavelength (2) of radiation has photons of energy (E) at 2.87 x 10 37
6.63X10 3459X
2.87X10
1.989X5sm
10. Â photon°ofvisible light has a frequency (v) of 4.3 x 10 S
2レ=6,934
入= obej
14 -1
What is the energy (E) of this
11. Calculate the energy( E) of a photon of radiation whose wavelength(^) is 322nm.
Note: (1nm=1x10-9-m)
-16
J?
2. What wavelength(^) of radiation has photons of energy (E) at 2.87 x 10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning