11. In which pair of compounds should the first member be more covalent than the second member? (A) TICI, TICI, LiF, BF3 (D) SnF4, CF4 (B) SnI4, SnF4 (C)
11. In which pair of compounds should the first member be more covalent than the second member? (A) TICI, TICI, LiF, BF3 (D) SnF4, CF4 (B) SnI4, SnF4 (C)
Chapter8: Bonding: General Concepts
Section: Chapter Questions
Problem 7RQ: Define formal charge and explain how to calculate it. What is the purpose of the formal charge?...
Related questions
Question
I need help on questions 11-12? Could you explain which option is correct?
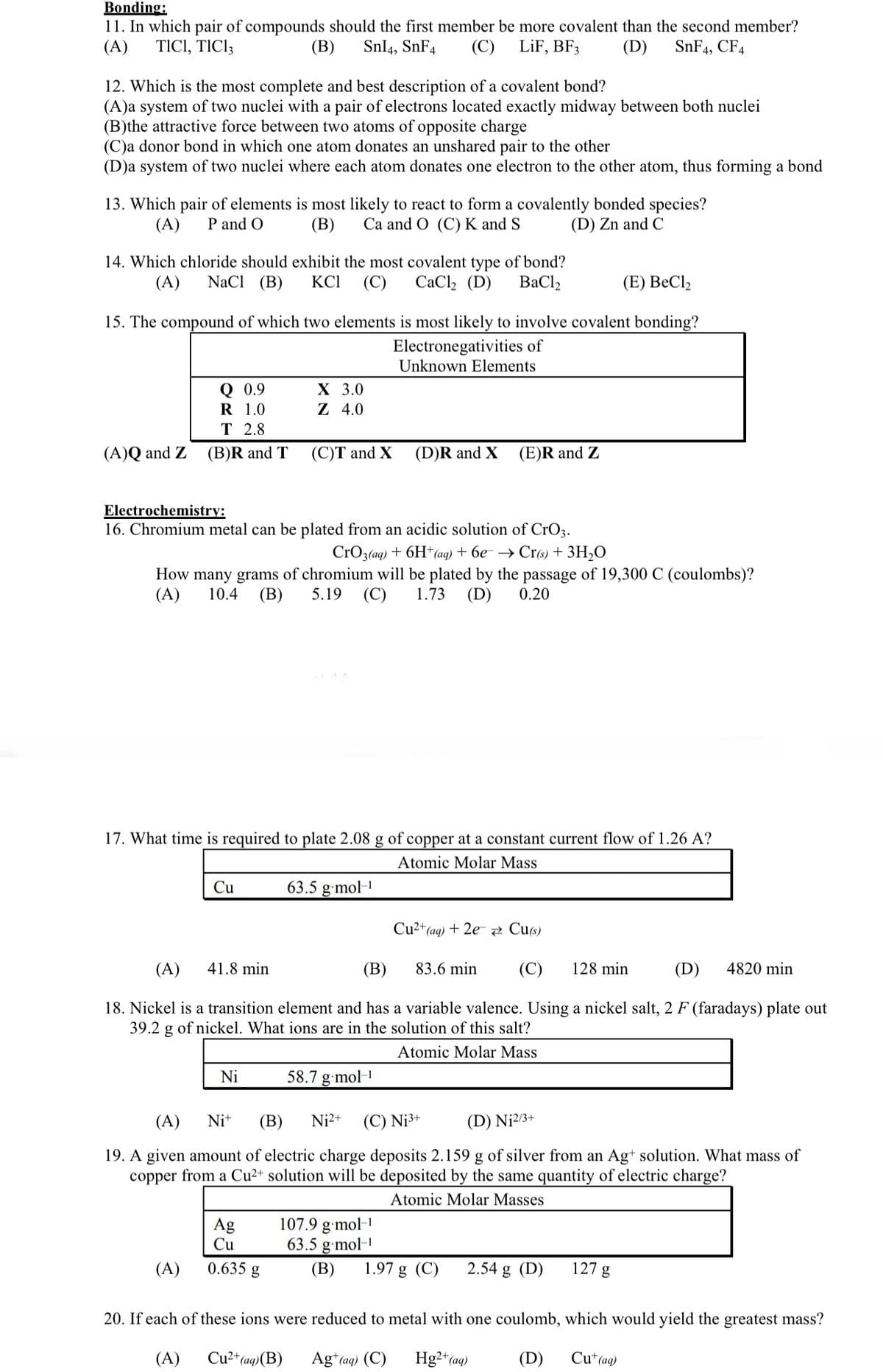
Transcribed Image Text:Bonding:
11. In which pair of compounds should the first member be more covalent than the second member?
(A) TICI, TIC13
(B) SnI4, SnF4 (C) LiF, BF3
(D) SnF4, CF4
12. Which is the most complete and best description of a covalent bond?
(A)a system of two nuclei with a pair of electrons located exactly midway between both nuclei
(B)the attractive force between two atoms of opposite charge
(C)a donor bond in which one atom donates an unshared pair to the other
(D)a system of two nuclei where each atom donates one electron to the other atom, thus forming a bond
13. Which pair of elements is most likely to react to form a covalently bonded species?
(B) Ca and O (C) K and S
(A) P and O
(D) Zn and C
14. Which chloride should exhibit the most covalent type of bond?
(A) NaCl (B) KCI (C) CaCl₂ (D) BaCl,
(E) BeCl₂
15. The compound of which two elements is most likely to involve covalent bonding?
Electronegativities of
Unknown Elements
(A)Q and Z
Q 0.9
1.0
R
T 2.8
(B)R and T
Cu
(A)
X 3.0
Z 4.0
Electrochemistry:
16. Chromium metal can be plated from an acidic solution of CrO3.
CrO3(aq) + 6H+ (aq) + 6e → Cr(s) + 3H₂O
How many grams of chromium will be plated by the passage of 19,300 C (coulombs)?
(A) 10.4 (B) 5.19 (C) 1.73 (D) 0.20
(C)T and X
17. What time is required to plate 2.08 g of copper at a constant current flow of 1.26 A?
Atomic Molar Mass
Ni
Ag
Cu
0.635 g
63.5 g.mol-¹
(D)R and X
(A) 41.8 min
(B)
83.6 min (C) 128 min
(D) 4820 min
18. Nickel is a transition element and has a variable valence. Using a nickel salt, 2 F (faradays) plate out
39.2 g of nickel. What ions are in the solution of this salt?
Atomic Molar Mass
(E)R and Z
58.7 g.mol-1
(A)
Ni+ (B) Ni²+ (C) Nj³+
(D) N₁2/3+
19. A given amount of electric charge deposits 2.159 g of silver from an Ag+ solution. What mass of
copper from a Cu²+ solution will be deposited by the same quantity of electric charge?
Atomic Molar Masses
107.9 g.mol-¹
63.5 g.mol-¹
(B)
Cu²+ (aq) + 2e Cu(s)
1.97 g (C) 2.54 g (D)
127 g
20. If each of these ions were reduced to metal with one coulomb, which would yield the greatest mass?
Cu²+ (aq) (B)
Hg2+ (aq)
Cu+ (aq)
(A)
Ag+ (aq) (C)
(D)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Step 1: Fajan's rule
VIEWStep 2: Determination of covalent character of first pair of compound.
VIEWStep 3: Determination of covalent character of second pair of compound.
VIEWStep 4: Determination of covalent character of third pair of compound.
VIEWStep 5: Determination of covalent character of fourth pair of compound.
VIEWSolution
VIEWTrending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning