Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter19: The Representative Elements
Section: Chapter Questions
Problem 26E
Related questions
Question
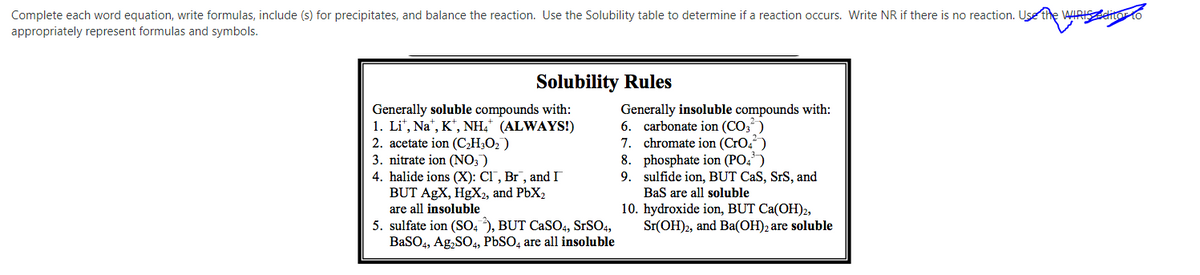
Transcribed Image Text:Complete each word equation, write formulas, include (s) for precipitates, and balance the reaction. Use the Solubility table to determine if a reaction occurs. Write NR if there is no reaction. Use the WIRISeitorto
appropriately represent formulas and symbols.
Solubility Rules
Generally soluble compounds with:
1. Li", Na", K*, NH,* (ALWAYS!)
2. acetate ion (C,H;O2)
3. nitrate ion (NO; )
4. halide ions (X): Cl, Br, and I
BUT AgX, HgX2, and PbX2
are all insoluble
Generally insoluble compounds with:
6. carbonate ion (CO3)
7. chromate ion (CrO,“)
8. phosphate ion (PO,')
9. sulfide ion, BUT CaS, SrS, and
BaS are all soluble
10. hydroxide ion, BUT Ca(OH)2,
Sr(OH)2, and Ba(OH)2 are soluble
5. sulfate ion (SO, ), BUT CaSO4, SrSO4,
BaSO4, Ag,SO4, PbSO, are all insoluble
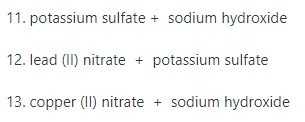
Transcribed Image Text:11. potassium sulfate + sodium hydroxide
12. lead (II) nitrate + potassium sulfate
13. copper (II) nitrate + sodium hydroxide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co