11. Treatment of compound F with lithium diisopropylamide followed by cyclohexanone gives either G or H. G is formed if the aldehyde is added at -78°C whereas H is formed if the aldehyde is added at 0°C. Furthermore, treatment of G with lithium diisopropylamide at 0°C gives H. Explain these results. CH2=CHCHCN OCH₂CH₂OC₂H F H OH G HO 137 CN CCH=CH2 OCH2CH2OC2H5 PROBLEMS CH2CH COCH2CH₂OC2H5 CN
11. Treatment of compound F with lithium diisopropylamide followed by cyclohexanone gives either G or H. G is formed if the aldehyde is added at -78°C whereas H is formed if the aldehyde is added at 0°C. Furthermore, treatment of G with lithium diisopropylamide at 0°C gives H. Explain these results. CH2=CHCHCN OCH₂CH₂OC₂H F H OH G HO 137 CN CCH=CH2 OCH2CH2OC2H5 PROBLEMS CH2CH COCH2CH₂OC2H5 CN
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 72AP: Treatment of 1-bromo-2-deuterio-2-phenylethane with strong base leads to a mixture of deuterated and...
Related questions
Question
Full Mechanisms need draw steps by explanation good mechanism do it full computational mechanisms full
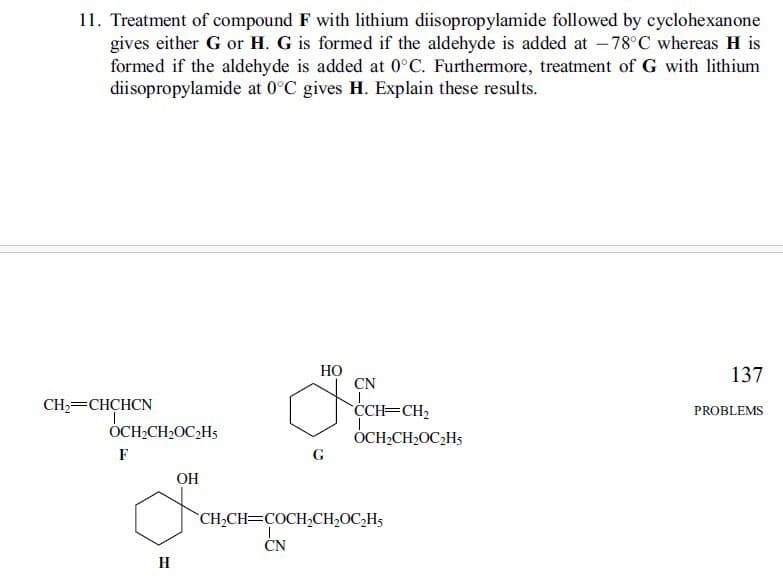
Transcribed Image Text:11. Treatment of compound F with lithium diisopropylamide followed by cyclohexanone
gives either G or H. G is formed if the aldehyde is added at -78°C whereas H is
formed if the aldehyde is added at 0°C. Furthermore, treatment of G with lithium
diisopropylamide at 0°C gives H. Explain these results.
CH2=CHCHCN
OCH₂CH₂OC₂H
F
H
OH
G
HO
CN
137
CCH=CH2
OCH2CH2OC2H5
PROBLEMS
CH2CH COCH2CH₂OC2H5
CN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 22 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning