11047Ag → 0.je + 110 48Cd For the nuclear reaction Starting with 200. g of Ag-110 in a container, how long will it take to decay to only 6.25 g ? The half-life of Ag-110 is 252 days. times. For each half-life we divide by 2. So, in order to reach 6.25 g we have to divide 200. g by 2 The number of half-lives is
11047Ag → 0.je + 110 48Cd For the nuclear reaction Starting with 200. g of Ag-110 in a container, how long will it take to decay to only 6.25 g ? The half-life of Ag-110 is 252 days. times. For each half-life we divide by 2. So, in order to reach 6.25 g we have to divide 200. g by 2 The number of half-lives is
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter21: Nuclear Chemistry
Section: Chapter Questions
Problem 34E: The isotope 208Tl undergoes decay with a half-life of 3.1 min.. (a) What isotope is produced by the...
Related questions
Question
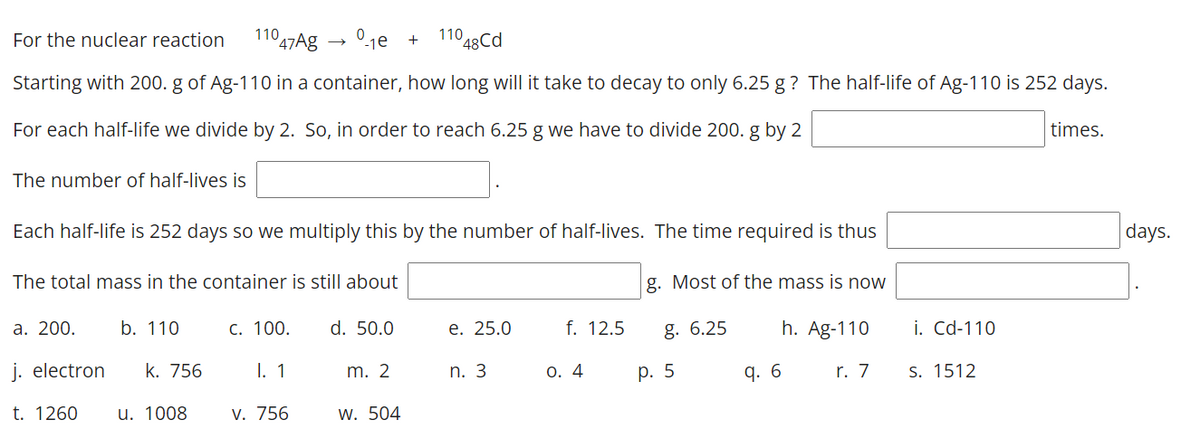
Transcribed Image Text:For the nuclear reaction
11047Ag → 0-1e +
110 48Cd
Starting with 200. g of Ag-110 in a container, how long will it take to decay to only 6.25 g? The half-life of Ag-110 is 252 days.
For each half-life we divide by 2. So, in order to reach 6.25 g we have to divide 200. g by 2
times.
The number of half-lives is
Each half-life is 252 days so we multiply this by the number of half-lives. The time required is thus
days.
The total mass in the container is still about
g. Most of the mass is now
а. 200.
b. 110
С. 100.
d. 50.0
е. 25.0
f. 12.5
g. 6.25
h. Ag-110
i. Cd-110
j. electron
k. 756
I. 1
m. 2
n. 3
О. 4
р. 5
q. 6
r. 7
S. 1512
t. 1260
u. 1008
V. 756
w. 504
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning