For the nuclear reaction 0.je + 110 48Cd 110 47Ag → Starting with 200. g of Ag-110 in a container, how many grams of Ag-110 remains after 756 days ? The half-life of Ag-110 is 252 days. The number of half-lives in 756 days is For each half-life we divide by 2. So, we divide 200. g by 2 times and there are g of Ag-110 remaining. The total mass in the container is still g. Most of the mass is now а. 200. b. 110 C. 100. d. 50.0 е. 25.0 f. 12.5 g. 6.25 h. Ag-110 i. Cd-110 j. electron k. 756 I. 1 m. 2 n. 3 О. 4 р. 5 q. 6 r. 7
For the nuclear reaction 0.je + 110 48Cd 110 47Ag → Starting with 200. g of Ag-110 in a container, how many grams of Ag-110 remains after 756 days ? The half-life of Ag-110 is 252 days. The number of half-lives in 756 days is For each half-life we divide by 2. So, we divide 200. g by 2 times and there are g of Ag-110 remaining. The total mass in the container is still g. Most of the mass is now а. 200. b. 110 C. 100. d. 50.0 е. 25.0 f. 12.5 g. 6.25 h. Ag-110 i. Cd-110 j. electron k. 756 I. 1 m. 2 n. 3 О. 4 р. 5 q. 6 r. 7
Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.18E
Related questions
Question
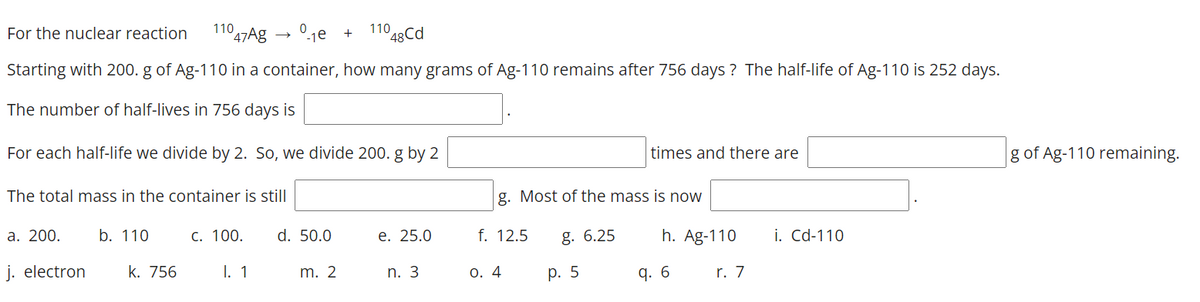
Transcribed Image Text:For the nuclear reaction
110
47Ag → 01e
1º48Cd
+
Starting with 200. g of Ag-110 in a container, how many grams of Ag-110 remains after 756 days ? The half-life of Ag-110 is 252 days.
The number of half-lives in 756 days is
For each half-life we divide by 2. So, we divide 200. g by 2
times and there are
g of Ag-110 remaining.
The total mass in the container is still
g. Most of the mass is now
а. 200.
b. 110
С. 100.
d. 50.0
е. 25.0
f. 12.5
g. 6.25
h. Ag-110
i. Cd-110
j. electron
k. 756
I. 1
m. 2
n. 3
0. 4
р. 5
q. 6
r. 7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning