12 60 70 8 9 10 110 13 140 3 2 5 QUESTION 2 What is the role played by NO in the following mechanism? 03 + NO → NO2 + 02 NO2 + O→ NO + O2 OA. spectator OB. product OC reactant OD. catalyst OE intermediate 10 4 15 160 17
12 60 70 8 9 10 110 13 140 3 2 5 QUESTION 2 What is the role played by NO in the following mechanism? 03 + NO → NO2 + 02 NO2 + O→ NO + O2 OA. spectator OB. product OC reactant OD. catalyst OE intermediate 10 4 15 160 17
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 110CP: Consider a reaction of the type aA products, in which the rate law is found to he rate = k[A]3...
Related questions
Question
Need help thx
Transcribed Image Text:Question comp
5
6
8
71
9 10
12
110
3
13
140 15
10
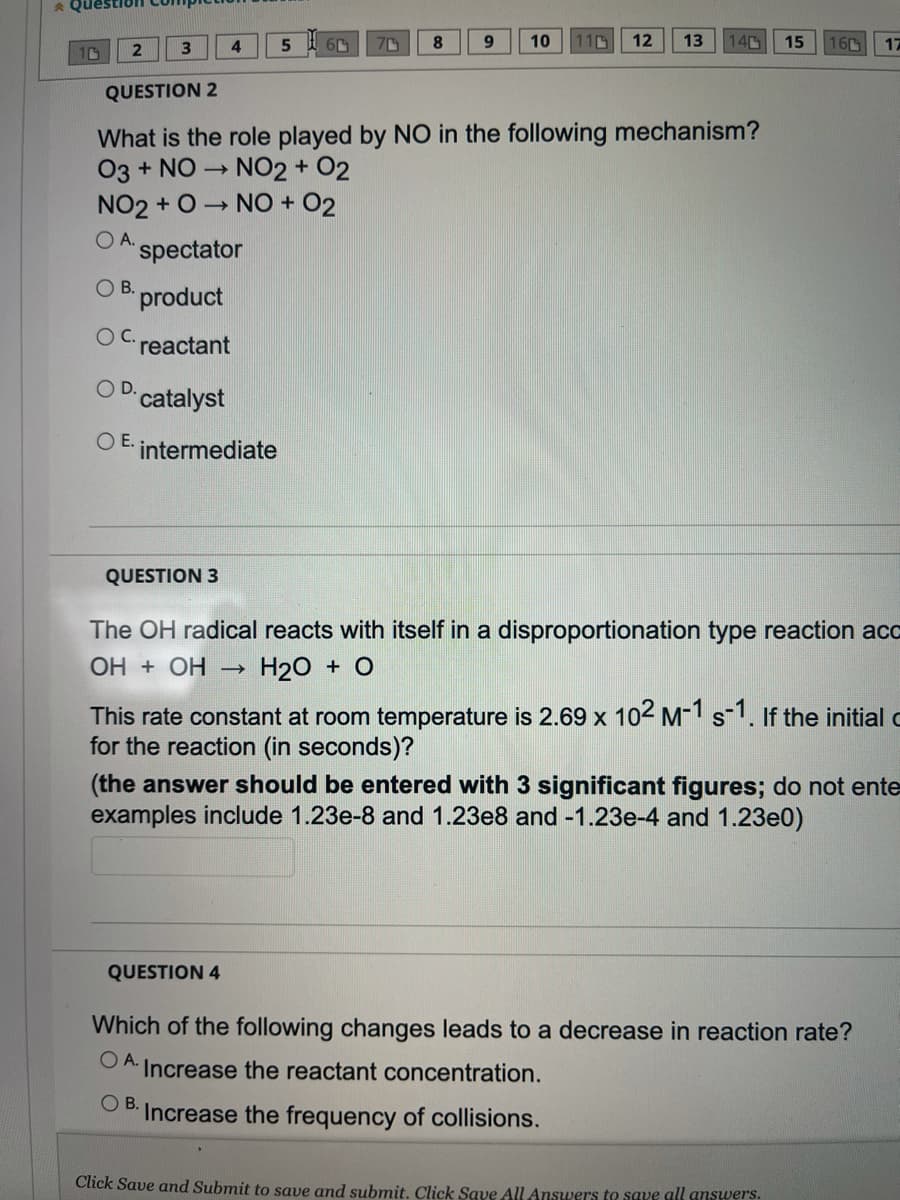
QUESTION 2
What is the role played by NO in the following mechanism?
03 + NO → NO2 + O2
NO2+ O→ NO + O2
OA. spectator
OB. product
reactant
O D. catalyst
OE. intermediate
QUESTION 3
The OH radical reacts with itself in a disproportionation type reaction acc
ОН + ОН – H₂O + O
This rate constant at room temperature is 2.69 x 10² M-1 s-1. If the initial c
for the reaction (in seconds)?
(the answer should be entered with 3 significant figures; do not ente
examples include 1.23e-8 and 1.23e8 and -1.23e-4 and 1.23e0)
QUESTION 4
Which of the following changes leads to a decrease in reaction rate?
OA. Increase the reactant concentration.
OB.
Increase the frequency of collisions.
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
4
160
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co