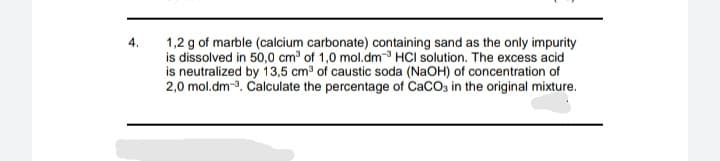
1,2 g of marble (calcium carbonate) containing sand as the only impurity is dissolved in 50,0 cm of 1,0 mol.dm HCI solution. The excess acid is neutralized by 13,5 cm of caustic soda (NAOH) of concentration of 2,0 mol.dm-3. Calculate the percentage of CaCOs in the original mixture.
Q: The pH of a 0.5L HNO2-NO2 buffer solution was determined to be 4.23. The solution was mixed with 5g…
A: Given, pH of buffer solution = 4.23 pKa = 4.35 Volume of buffer solution = 0.5 L weight of HNO2 = 5…
Q: 10. Based upon your observations of the dissolution of NH,Cl in exothermic or endothermic? Briefly…
A: We above to explain about enthalpy of dissolution of ammonium chloride
Q: 1. Titration of 0.485g sample by the Mohr method required 38.8mL of standard 0.1060 M AgNO3…
A: Given, Weight of sample= 0.485 g Concentration of AgNO3 = 0.1060 M Volume = 38.8mL = 38.8/1000 =…
Q: The distribution coefficient, k = concentration in hexanes concentration in water, between hexanes…
A: Amount of A in solution = 10 g
Q: Calculate the alkaline strength of pearl ash ( impure potassium carbonate) in terms of percent K2O…
A: Using the given date, Mass of Na2CO3 = 48.03×0.0053g Mass of KHC2O4 ·H2O = 2.02×0.02192g Mass of O…
Q: A mixture contains Na2CO3, NaOH and inert matter. A sample weighing 1.500 g requires 28.85 ml of…
A: Introduction: The given sample has a mixture of Na2CO3 and NaOH, here we use two indicators like…
Q: A solution (total volume = 1L) of 0.4 M NH3 contains 12.78 g of NH4CI (MW = 53.491 g/mol). Kb = 1.8…
A: First we would Calculate initial pH of solution before adding HCl. Then pH after adding HCl is…
Q: If K= 3.5 & K= 1.58 x 10 10, Suppose 100 ml of 0.010M aqueous amine is extracted with 200ml of…
A:
Q: The Ksp of Ca3(PO4)2 is 1.3 × 10−26. Estimate the solubility of this salt in units of g. L-1
A:
Q: Describe the preparation of I L of 5moll-2 HCL colution from concentrated acid/d=1019 g/m²: 37.500)
A: **As you have asked multiple questions, I am solving first question. Either resubmit other questions…
Q: A mixture of pure BaCO3 and pure Na2CO3 weighs 1.000 g and has the total neutralizing power of…
A:
Q: 3 L contaminated air 50 mL 0.0116 M in an air pollution analysis Carbon dioxide (CO2) BaCO3 is…
A:
Q: What mass of ZnS (Ksp = 2.5 x 10-22) will dissolve in 300. mL of 0.050 M Zn(NO3)2? Ignore the basic…
A: The mass of Zinc Sulfide has to be given, The balanced chemical equation is, Zns(s)⇔Zn2+(aq)+S2-(aq)…
Q: 1.217-g sample of commercial KOH, contaminated by Noel with K2CO3, was dissolved in water and the…
A: Let the KOH in the sample be y grams.Then, K2CO3 will be (1.217-y) g.
Q: In the MgO experiment, you made a buffer solution by combining 57 mL of 17 M NH3 with 7.0 grams of…
A:
Q: What is the final molar concentration of a 500.0ml solution prepared from 15.0 ml of a 12.0 M…
A: Since you have posted 3 completely unrelated questions, we are entitled to answer the first only.…
Q: mass of NaCH3COO must be dissolved in 400 mL of 0.46 M CH3COOH in order to prepare a solution with…
A:
Q: 5.31e-08 mol/L, what is the Ksp at this temperature? Ksp = (b) It is found that 9.14e-08 g of…
A:
Q: A saturated aqueous solution of Ni(OH)2 has a pH of 11.8. Calculate the Ksp of the solution and…
A:
Q: What is the molar solubility of calcium fluoride (Ksp = 3.9 x10-11) when dissolved in 150 mL in…
A: Since NaF is a strong electrolyte Hence it will dissolve completely into water Hence the…
Q: A sample of water at pH 10.5 has 75 mg/L of CO3-2 and 65 mg/L of HCO3- Including the contribution of…
A:
Q: A 0.2182g sample of NaCl was assayed by the Volhard Method using 50mL of 0.0985N AgNO3 and 11.75mL…
A: Given the mass of impure NaCl sample = 0.2182 g In Volhard's Method for the determination of…
Q: Water sample was observed to contain the following impurities (concentrations in ppm): CaCl2: 111,…
A: Since you have posted a question with the multiple sub parts, we will solve first subparts for you…
Q: For water having a total alkalinity of 1.00x10^-3 mol/L and a pH of 10.34 what is the percentage…
A: Given: Total alkalinity of water = 1.00 × 10-3 mol/L pH = 10.34
Q: A 25.00-mL sample of a household cleaning solution was diluted to 250.0 mL in a volumetric flask. A…
A: Answer: This is a question based on acid-base titration where base is the analyte and acid is the…
Q: An alkaline sample of sodium compounds weighing 1.196 g was dissolved in water, cooled at 15 deg C,…
A: Alkaline compounds of sodium may involve sodium hydroxide (NaOH), sodium bicarbonate (NaHCO3) and…
Q: 1. Calculate the solubility of BaSO4 in a solution that is 0.01 and 0.05 M relative to NaCl and KCI,…
A: BaSO4 will be having no common ions in NaCl and KCl. Hence, the solubility of BaSO4 will be its own…
Q: 4.912-g sample of a petroleum product was burnedin a tube furnace, and the SO2produced was…
A:
Q: which is tribasic(H3Y). 25cm^3 of lemon juice was diluted to 250cm^3. 25cm^3 of the solution was…
A: The question is based on the concept of solutions. we have to quantitatively estimate the citric…
Q: Quantitatively compare the solubility of AgBr in H2O and 1.0M NH3. Ksp AgBr=5.4x10-11 and…
A:
Q: One strategy for dealing with the acidification of lakes is periodically to add powered limestone…
A: Solution - According to the question - Given - Ksp expression for CaCO3 is Ksp = [Ca2+] [CO32-]…
Q: Condition of permanganatometric titration of sodium oxalate includes: pH = 1 and heating of analyte…
A: Permanganatometric titration is a technique used in chemical quantitative analysis. It is a type of…
Q: The distribution coefficient, k = concentration in hexanes concentration in water, between hexanes…
A: The extent of the solubility of two immiscible components in two different phases is called the…
Q: Describe the preparation of the following solutions: a. 3.50 L of 0.500 M KSCN from the solid…
A:
Q: n alkaline sample of sodium compounds weighing 1.196 g was dissolved in water, cooled to 15°C,…
A: Given: Mass of alkaline sample of sodium compounds= 1.196 g Normality of H2SO4= 1.058 N The volume…
Q: 1) Assuming that 0.433 g of precipitate is recovered calculate the percent by mass of SO42− in the…
A: Molar mass of sulphate, SO42- = 32.1 +4×16 = 96.1 g/mol Given Data: Mass of unknown sulfate salt =…
Q: Calculate the concentration of Ca**, Cl- in mM/L and meq/L; and hardness in mg/L as CaCO3 produced…
A: Hardness is expressed as milligrams of calcium carbonate equivalent per litre. Calcium carbonate is…
Q: A 370.00 mL solution of 0.00190 M AB5 is added to a 200.00 mL solution of 0.00165 M CD2. What is…
A: According to the mole concept, in terms of mass, the amount of substance in moles is equal to the…
Q: What mass of solid Lanthanum (III) oxalate nonahydrate { La2 ( C2O4 )3 ∙ 9 H2O } can be obtained…
A:
Q: 25 g of the dilute mixture of H2SO4 and HNO3 is completed to 500 mL with water. When BaCl2 is added…
A: First, we need to calculate the amount of H2SO4 then we will find the amount of HNO3.
Q: Give a list of laboratory apparatus and equipment that are to be utilized for the preparation of 500…
A:
Q: A 3.026 grams of Cu (II) salt is dissolved in water and then diluted to 250 mL in a volumetric…
A:
Q: describe in detail the preparation of 800 mL of 45 parts per thousand Cl- from 0.01 M P6CI2
A: The concentration is parts per thousand (ppt) describes that 1 g of solute is present in the 1 L of…
Q: Calcium phosphate (Ca3(PO4)2; MM = 310.18 g/mol), a large component of human bone of 2.07 x 10-33 at…
A:
Q: Sulfur containing compound weighing 4.8670 grams was digested and purified to form H,SO4. A 25.00-mL…
A: Moles = Molarity * Volume (L) Mass = Moles * Molar mass
Q: Enough silver sulfite (Ag2SO3 , Ksp = 1.5 × 10-14 ) is dissolved in 2.00 L to produce a saturated…
A: Given: pH = 2.00 And volume of solution = 2.00 L Concentration of H+ = 10-pH = 10-2.00 = 1.0 × 10-2…
Step by step
Solved in 3 steps

- Calcium fluoride is considered as a relatively insoluble compound and therefore lime or slakedlime has been considered as a possible material to remove excess fluoride in water of boreholesin certain parts of the country. The solubility product of CaF2 is Ksp = 3 x 10 – 11 and that ofCa(OH)2 isKsp =8x10-61. How much lime can be added to the water to remove 10 mg of F- ion per litre ofborehole water?(The atomic masses are Ca: 40.08; F: 19.00; O: 16; H: 1)A 0.2182g sample of NaCl was assayed by the Volhard Method using 50mL of 0.0985N AgNO3 and 11.75mL of 0.1340N NH4SCN. Calculate the %NaCl in the sampleIt is required to know the concentration of an aqueous solution of H2SO4 that appeared in the laboratoryChemistry III and it's unlabeled. To this end, a student of analytical chemistry carried out the followingProcedure: He took 5.00 mL of a fresh and standardized solution of 0.525M NaOH and brought them to a250.0 mL balloon to be completed with distilled water. Subsequently, he poured 15.00 mL of the solutionH2SO4 of unknown concentration in an Erlenmeyer flask and added 2 drops of phenolphthalein.Using a burette filled with the last NaOH solution, he noticed that when adding 39.40 mL of the hydroxidethe Erlenmeyer solution reached a faint but permanent pink. With the above dataDetermine the concentration and pH of the H2SO4 solution.
- How many grams of CaF2 (molar mass = 78.077) will dissolve in 650 mL of 0.15 M NaF solution? The Ksp for CaF2 is 3.8904e-11.A mixture of pure BaCO3 and pure Na2CO3 weighs 1.000 g and has the total neutralizing power of 15.37 meq of CaCO3. Calculate the percentage of combined CO2 in the mixture and the weight of Li2CO3 that has the same neutralizing power as 1.000 g of the above mixture.Approximately 6.0mL of concentrated perchloric acid (70%) was transferred to a bottle and diluted with about 1.0L of water. A sample containing 251.5 mg of primary standard Na2B4O7.10H2O required 27.41 mL of the HClO4 solution to reach the methyl red end point. Calculate the molar concentration of the HClO4 (Fwt = 382g/mole)
- 0.1 g of the mixture of na2so4 and k2so4 is taken and 100 ml of solution is prepared. 10 ml of this prepared solution is placed in a beaker and some distilled water is added. A mass of 15.5 mg is obtained by precipitation with Bacl2 at PH=5, then filtering and bringing to a constant weight at 800 °C. Calculate the percentages of Na2so4 and K2so4 in the mixture accordingly.A sample containing calcium was analyzed by gravimetry, converting all calcium to calcium oxalate monohydrate (CaC2O4 • H2O). The sample weighing 0.2654 g was dissolved by adding 6M HCl, and then ammonium oxalate solution was added to precipitate all the calcium. The precipitate was suction filtered and dried in an oven at 95 °C for one hour. The solid obtained weighed 0.3216 grams.Calculate:A) The percentage of CaCO3B) The percentage of Ca C) The percentage of CaOCalculate the solubility of BaSO4 with the Ksp = 1.08 x 10-10 ,in an aqueous solution with I = 0.0010 mol kg-1
- Dissolved 0.273 grams of pure sodium oxalate (NaCO) in distilled water and added sulfuric acid and titration the solution at 70 ° C by using 42.68 ml of KMNO. solution and has exceeded end point limits by using 1.46 ml of standard oxalic acid (HCO) with 0.1024 N Calculate the normlity of KMNO Note that the molecular weight of sodium oxalate (NaCO) = 134 and its equivalent weight = 67A 600.0 mg sample consisting of only CaC2O4and Mg C2O4is heated at 500oC converting the two salts to CaCO3and MgCO3. The sample weighs 465.0 mg. If the sample had been heated at 900oC where the products are CaO and MgO, what would the mixture of oxides weigh? Show the clear and complete solutionThe solubility of AX2 is 0.000047 M What is the Ksp of AX2?



