12. The solubility-product constant for K2PDC16 is 6.0 x 106 (K2PDCI6 2 2K* + PDCI?-). What is the K* concentration of a solution prepared by mixing 50.0 mL of 0.200 M KCl with 50.0 mL of a. 0.0500 M PdCI?-? b. 0.100 M PdCI?-? c. 0.200 M PdCI?¯?
12. The solubility-product constant for K2PDC16 is 6.0 x 106 (K2PDCI6 2 2K* + PDCI?-). What is the K* concentration of a solution prepared by mixing 50.0 mL of 0.200 M KCl with 50.0 mL of a. 0.0500 M PdCI?-? b. 0.100 M PdCI?-? c. 0.200 M PdCI?¯?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.4: Solubility Of Salts
Problem 4RC: 4. What is the solubility of PbSO4 in water at 25°C if the solution already contains 0.25 M...
Related questions
Question
100%
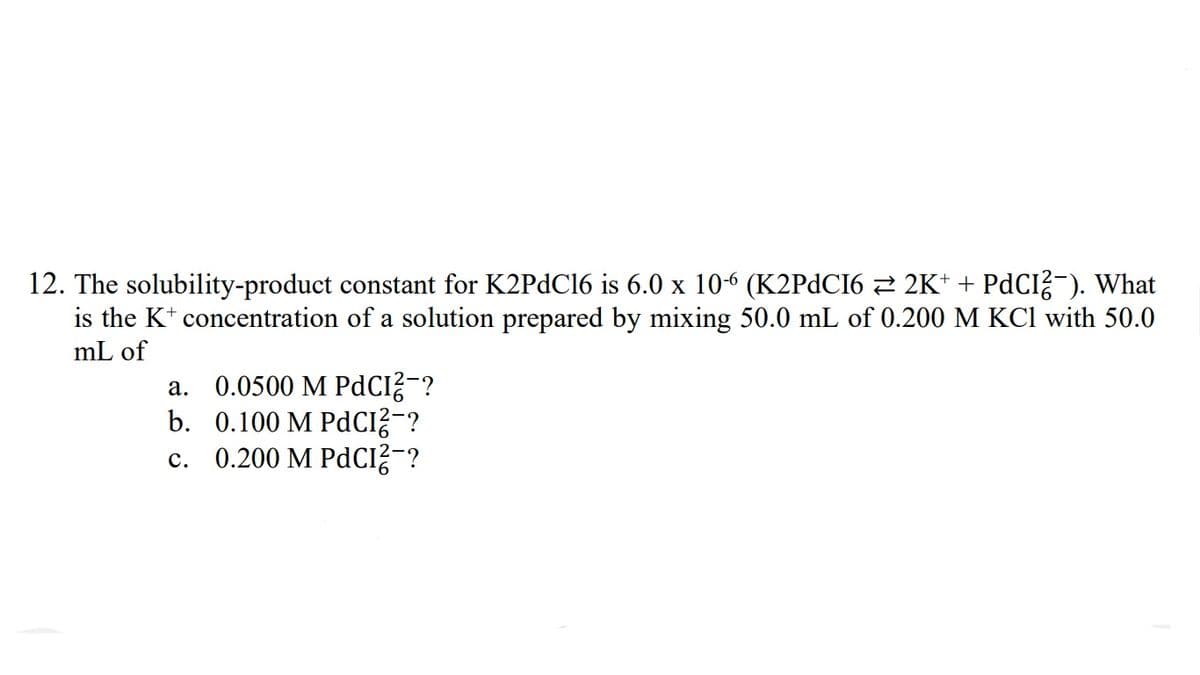
Transcribed Image Text:12. The solubility-product constant for K2PDC16 is 6.0 x 10-6 (K2PDCI6 2 2K* + PdCI?-). What
is the K* concentration of a solution prepared by mixing 50.0 mL of 0.200 M KCl with 50.0
mL of
a. 0.0500 M PdCI?-?
b. 0.100 M PdCI?¯?
c. 0.200 M PdCI?¯?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning