123 g of water is placed in an insulated cup. It’s initial temperature is 25.6°C. A 587.0 g Sample of a nonmetal is heated. It’s initial temperature is 125.0°C. The hot metal is added to the cold water. The final temperature of both is 39.3°C. Determine the specific heat of metal, identify the metal ?
Q: ou place 300.0 grams of water in a coffee cup calorimeter and the temperature of the water is 22.3℃.…
A: answer = 23.65 0C
Q: If Water has a specific heat of 4.184 J/g°C. How much heat is required to increase 10.0 g of water…
A:
Q: A 125-g piece of aluminum (specific heat of 0.900 J/gC) is heated to 95°C and placed into a 375-g…
A: Solution:- Mass (m) of aluminium piece = 125 g Specific heat capacity (ca) of aluminium = 0.900…
Q: A gas in a closed container is heated, causing the lid of the container to rise. The gas performs 3J…
A: Given: work done ( w) = - 3 J Internal energy change ( ∆U ) = + 15 J Heat absorbed ( q ) = ? By…
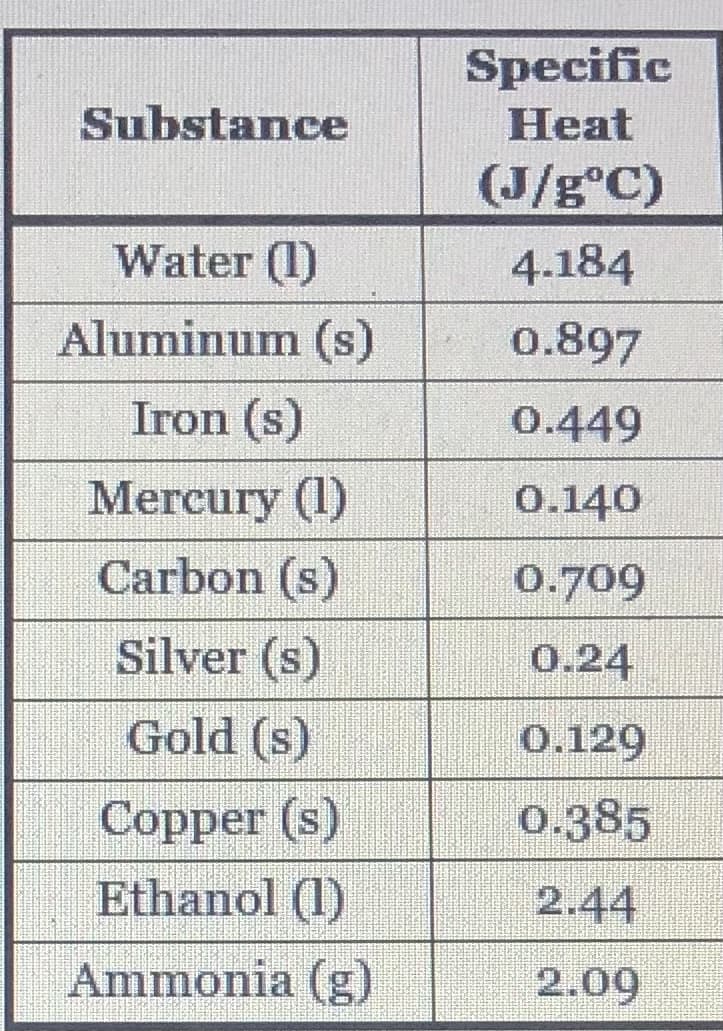
Q: The specific heat capacities for several substances are shown in the table. Substance Specific Heat…
A: We have to predict which substance wil undergo greatest temperature change .
Q: A 35.0-g sample of water at 25°C is mixed with 45.0 g of water at 88°C. Calculate the final…
A:
Q: A 10 g piece of copper at 145 oC is placed in 150 mL of water. The initial temperature of water is…
A:
Q: A chemist heats the block of copper, then places the metal sample in a cup of oil at 25.00 C instead…
A: Since the final temperature of oil will be same as that of copper block as they reached equilibrium…
Q: A sample of copper absorbs 43.6 kJ of heat, resulting in a temperature rise of 40.0°C, determine the…
A: From thermodynamics: q=m.c.∆T . . . (1) Here, q is the…
Q: A 75.0 gram sample of a metal is heated until it is at 100.0℃. A calorimeter contains 100.0 grams of…
A: Given that : Mass of sample of the metal (ms)= 75.0 g Its temperature = 100°C Mass of water in the…
Q: A 44.3 g sample of water at 100.00 °C (specific heat of water = 4.184J/g.°C) was placed in an…
A:
Q: The specific heat of AI is 0.901 J/g C. The specific heat of Iron is 0.449 J/g C. Which substance…
A: The quantity of energy (Q) required can be calculated as follows,
Q: Consider the following specific heats of metals. Metal Specific Heat Aluminum 0.897 J/°C∙g…
A:
Q: A metal object with mass of 29.0 g is heated to 97.0 °C and then transferred to an insulated…
A: Since the heat lost by metal + heat gain by water should be = 0 as no heat is lost in surroundings…
Q: A student places a block of hot metal into a coffee cup calorimeter containing 151.0 g of water. The…
A: Given data,Mass of water=151.0gInitial temperature of water=23.3oCFinal temperature of…
Q: What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased…
A: The specific heat of a substance is defined as the amount of heat required to raise the temperature…
Q: A 625 g chunk of iron is removed from an oven and plunged into 525 g of water in an insulated…
A: The heat lost by iron = heat gained by water heat gained by water (q) = m x S x ΔT…
Q: A 35.0-g sample of water at 25°C is mixed with 45.0 g of water at 88°C. Calculate the final…
A: Calculate for the final temperature of the mixture see step-2 for further Calculation and concept…
Q: A metal object with the mass of 24.1 g is heated to 97.0 *C and then transferred to an insulated…
A: Given: Specific heat of water = 4.18 J/g. oC Mass of metal = 24.1 g Mass of water = 86.9 g. Initial…
Q: Quantity Mass of can (g) 12.695 mass of can and water (g) 125.129 Initial mass of food sample (g)…
A:
Q: A metal object with a mass of 19 g is heated to 96°C and is transferred to a calorimeter containing…
A: Given data: The mass of metal = 19 g. The temperature of the metal = 96°C. The mass of water = 75…
Q: Aluminum has a specific heat of 0.902 J/goC. How much heat is gained when a piece of aluminum with a…
A:
Q: A piece of unknown metal weighs 217 g. When the metal piece absorbs 1.43 kJ of heat, its temperature…
A: Given: Mass of metal, m = 217 g Heat energy, q = 1.43 kJ Initial temperature, T1 = 24.5oC Final…
Q: A 25.0 g sample of silver metal was heated to 170 degrees C and then placed in a calorimeter holding…
A: Given: Mass of silver metal = 25.0 g The initial temperature of the metal= 170°C Mass of water= 50.0…
Q: A 192-gram piece of copper was heated to 100.0oC in a boiling water bath, and then it was dropped…
A: According to law of calorimetry heat absorbed = heat released. Copper has higher temperature so it…
Q: Determine the specific heat capacity of 250.0g of an unknown metal. When the metal absorbs 18.1 kJ…
A: a) Given that: mass of metal = 250.0g initial temperature, Ti = 23.1°C final temperature, Tf =…
Q: A block of aluminum of unknown mass has an initial temperature of 58.6°C. The aluminum is immersed…
A:
Q: A 90.0 g piece of metal, initially at 98.6°C, is placed into 120.0 g of water initially at 24.3°C.…
A:
Q: A 90.0 g piece of metal, initially at 98.6°C, is placed into 120.0 g of water initially at 24.3°C.…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: A student measures the following data regarding the heat of fusion of ice: 24.8 g ice at 0.0°C is…
A: Given: Heat capacity of calorimeter = 15.4 J/oC Initial temperature of calorimeter and water = 21.5…
Q: A beaker of hot water with a volume of 43.9 mL has an initial temperature of 94.3 °C. You place a…
A:
Q: How much energy is needed to raise the temperature of 325g of water by 10.0 degrees? The specific…
A: Thermodynamics is branch of chemistry in which we deal with heat of reaction.
Q: A 350g sample of aluminum is taken out of the freezer at –20oC and placed into 500g of water at 14.6…
A: Given Mass of water = 500 g Initial temperature = 14.6°C Final temperature = 10°C Mass of…
Q: Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500…
A: Interpretation: The specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs…
Q: A 77.5g piece of brass is heated so that its temperature is 98.7◦C. Next, it is quickly placed in a…
A:
Q: A metal object with mass of 20.9 g is heated to 97.0 °C and then transferred to an insulated…
A:
Q: Exactly 385 J will raise the temperature of 76 g of a metal from 15.7oC to 50.9oC. What is the…
A: The given data: The mass of metal is 76 g The temperature of metal raises from 15.70C to 50.90C…
Q: An unknown solid metal at 150 degrees celsius was placed in 1.00 L of water at 25 degrees celsius.…
A: Heat absorbed or emitted by a substance = mass of the substance x specific heat of the substance x…
Q: A coffee cup calorimeter, including that water it contains, has a heat capacity of 425 J/K, and is…
A: Formula: q = C × ∆T where , q = heat C = Heat Capacity ∆T = Change in temperature The Heat lost…
Q: In the laboratory a student determines the specific heat of a metal. He heats 18.4 grams of…
A:
Q: In the lab you heat a 100.0 g sample of lead from 22oC to 80oC and add it to a 100-gram sample of…
A: The heat capacity is the ratio of the energy supplied in the form of heat to the rise in the…
Q: If 80.0 g of aluminum initially at 70˚C is dropped into 250.0 mL of water the temperature of the…
A:
Q: An unknown hot metal at 107.03°C with a mass of 48.67g was mixed with 37.99g of water at an initial…
A: Given data contains, Mass of unknown metal , mm= 48.67 gInitial temperature , Tim= 107.03°CMass of…
Q: A 110.-g sample of copper (specific heat capacity = 0.20 J/°C g) is heated to 82.4°C and then placed…
A: Given : Mass of copper = 110 g Specific heat capacity of copper = 0.20 J/°C.g Initial temperature of…
Q: Aluminum and iron samples, each weighing 2 kg, absorb 11.5 kJ of heat. Which material would have the…
A: The amount of heat required to increase the 1oC temperature of 1 g metal is known as the specific…
Q: A metal object with a mass of 19g is heated to 96 degrees C and then transferred to a calorimeter…
A: The amount of heat absorbed (or released) by fixed amount of a substance to change its temperature…
Q: A sample of copper absorbs 43.6 kJ of heat, resulting in a temperature rise of 75.0°C, determine the…
A: Heat (q) = 43.6 KJ ....Or (because, 1 KJ = 1000 J). So, = 43.6 KJ × (1000 J / 1KJ) = 43600 J.…
Q: A group of students place 15.41g of a metal into a test tube. They place the test tube into a beaker…
A: Given, mass of the metal = Wmetal = 15.41 g Mass of the water = Wwater = 10.47 g Initial…
Q: 10. It took 4.15 x 103 J of energy to raise the temperature of a piece of copper metal from 31°C to…
A: We have to calculate the mass of metal.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- 4.After the sinking of RMS Titanic, US operated the International Ice patrol with the purpose of monitoring the presence and movements of icebergs in the Atlantic and Arctic oceans. Once, they observed a unique tabular iceberg in a form of a rectangle (250 km x 50 km x 300 m). How many years would it take to melt the iceberg solely by heat from the sun if the ice absorbs a mean of 98 J/m2-s, 12.5 hrs per day? Note: the exposed area for heating is 250 km x 50 km and the density is 920 kg/m3.You have the six pieces of metal listed below, plus a beaker of water containing 300 g of water. The water temperature is 21.0 °C and its specific heat is 4.184 J/g⋅°C. Metal Specific heat (J/g⋅°C) Mass (g) 1. Al 0.9002 100.0 2. Al 0.9002 50.0 3. Au 0.1289 100.0 4. Au 0.1289 50.0 5. Zn 0.3860 100.0 6. Zn 0.3860 50.0 In an experiment, you are asked to select one piece of metal and heat it to 100.0 °C, and then select a second piece of metal and cool it to ‒10.0 °C. Both pieces of…A brick lies perilously close to the edge of the flat roof of a building. The roof edge is 50 ft above street level, and the brick has 510.0 J of potential energy with respect to street level. Someone edges the brick off the roof, and it begins to fall. What is the brick’s kinetic energy when it is 35 ft above street level? What is the brick’s kinetic energy the instant before it hits the street surface?
- A student investigates the enthalpy of solution, ΔHsoln for ammonium chloride, NH4Cl. In addition to the salt, the student has access to a calorimeter, a balance with a precision of ±0.1 g, and a thermometer with a precision of ±0.1°C. To measure ΔHsoln for NH4Cl, the student adds 904.0 g of water, initially at 31.0 °C to a calorimeter and adds 155 g of NH4Cl (s), stirring to dissolve. After the NH4Cl dissolves completely, the temperature of the solution is 90.0 °C. Assume the calorimeter was perfectly insulated and the specific heat of the solution was 4.2 J/(g·K). (a) Calculate the q for the dissolving of NH4Cl in water. Include units and proper sign in your answer. Show all work. (b) Calculate the ΔHsoln for NH4Cl, in kJ/molrxn. Include units and proper sign in your answer. Show all work. (c) Is the dissolving of NH4Cl endothermic or exothermic? Justify your answer.A chunck of lead at 91.7 degrees celcius was added to 200.0 g of water at 15.5 celcius. the specific heat of lead is .129 J/gC, and the specific heat of water is 4.18 J/gC. when the temperature stabilized, the temperature of the mixture was 19.4 celcius. assuming no heat was lost to surroundings, what was the mass of lead added? a. 1.73 kg b. 350 g c. 332 g d. 276 g e. none of these thanks for your time and help :)What is the enegry numbers for the table?1 calorie (cal) = 4.184 J1 kWh = 3.600 × 106 J
- An important flavor component of vanilla extract is vanillin (C8H8O3, molar mass = 152.15 g/mol). When vanillin burns in a bomb calorimeter with a heat capacity of 7.87 kJ/°C, the temperature increases from 25.52°C to 30.43°C. If the heat of combustion of vanillin is -3,760 kJ/mol, what mass of vanillin (in g) was combusted? Please give your answer to 2 decimal places.TRUE OR FALSE 1. Heat flow is a directional quantity. Heat flows from a warmer body to a colder body. 2. The SI unit of heat is calorie.A solution is made by mixing 232.0 mL of ethanol initially at 12.7 ∘C with 232.0 mL of water initially at 22.5 ∘C. What is the final temperature of the solution assuming that no heat is lost? The density of ethanol is 0.789 g/mL and the density of water is 1.00 g/mL. The specific heat of ethanol is 2.46 J/g·°C and the specific heat of water is 4.184 J/g·°C ?f= ∘C
- A list of the calorie content of foods indicates that a 10 oz chocolate thick shake contains 333 Calories. Express this value in kJ and in J. The Joule (J) is the SI unit of energy. 1 calorie (cal) = 4.184 J; 1 Calorie (Cal) = 1000 cal = 1 kcal kJ_____ J______The specific heat of gold is 0.13 J/gC and copper is 0.39 J/gC. Suppose we heat a 25 g sample of gold and a 25 g sample both starting at 25 degrees C to 80 degrees C. They are each individually dropped into separate beakers containing 100 grams of water at 10 degrees C and allowed to sit until the temperature stabilizes. Which of the following statements is true? Both beakers of water will be the same temperature The beaker containing gold will be a higher temperature The beaker containing copper will be a higher temperaturean important flavor component of vanilla extract is vanillin C8H8O3 molar mass = 152.15 g/mol when vanillin burns in a bomb calorimeter with a heat capacity of 8.60 kJ/°C the temp increases from 25.97°C to 31.18°C. if the heat of combustion of vanillin is -3760 kJ/mol what mass of vanillin (in g) was combusted? answer to two decimal places

